|
Introduction
1% Tropicamide eye drop and 2% Homatropine eye drops are commonly used eye drops in day to day ophthalmic practice. Tropicamide is an acetylcholine receptor blocker drug, which has short acting cycloplegic and mydriatic effect. Homatropine belongs to Anticholinergic group which has more potent cycloplegic effect then Tropicamide. Both the drugs having known effect on the axial parameters of the eye like anterior chamber depth (ACD),lens thickness (LT), vitreous chamber length (VCL), and axial length (AXL) of the eye.
ACD measurement provides valuable information in different fields of ophthalmology. In cataract surgery and phakic intraocular lens [IOL] implantation, precise ACD measurements are required to determine IOL power and position and to prevent endothelial cell damage.1 ACD measurement assessment can also provide an indicator of glaucoma, with the anterior chamber being shallower in patients at risk.
Different devices capable of measuring the ACD are based on variety of techniques and can classified accordingly. The most commonly and widely used method is the A-Scan ultrasonic method and its measurements of ACD and other axial parameters represent the "Gold Standard" for biometric dimension.1 The accuracy of a measuring instrument is an essential factor when selecting a device for clinical purpose. The purpose of this study was to demonstrate the effect on axial parameters of the eye, after instillation of 1% Tropicamide eye drop and 2% Homatropine eye drop.
Methods
This study was conducted in the out-patient department of Ophthalmology. The A-Scan used to measure the anterior chamber depth, lens thickness, vitreous chamber length, and axial length, was the Alcon ultrasonic scan using a frequency of 10 MHz.
Measurement of the effects of topically instilled 1% Tropicamide (76 eyes) and 2% Homatropine eye drop (28 eyes) was done in patients who sought treatment for their eye problems. All the patients included in this study were informed of the nature of the study and had given verbal consent to participate. Complete ocular examination was done in each case, beforehand, to rule out any other ocular disease affecting the procedure. The exclusion criteria from the study was, cases of glaucoma, shallow anterior chamber, deep anterior chamber, past history of ocular surgery, patients with high refractive error (more than 2 Diopter),use of contact lens, patients below 10 years and above 45 yrs, pregnancy, diabetic patients, and lack of co-operation.
The base line reading before the instillation of 1% Tropicamide and 2% Homatropine was done which included assessment of anterior chamber depth, lens thickness, vitreous chamber length, and overall axial length of the eye. The anesthesia required for A-Scan was gained by instilling one drop of 0.5% Proparacaine hydrochloride in each eye.
One drop of tropicamide and Homatropine was instilled twice at the interval of 15 minutes in respective group patients. The reading 45 minutes after instillation of the 1% Tropicamide and 60 minutes after the instillation of Homatropine were done using the same parameters. All the measurements were made in straight ahead position using ultrasonic transducer.
Results
A total of 76 eyes with Tropicamide and 28 eyes with Homatropine were examined. The mean age of the subjects was 24.7 years in the Tropicamide group while 12.1 years in Homatropine group. The limit of agreement was used as the indicator of the similar trend seen in the patients on various A-scan parameters after using tropicamide or homatropine eye drops in the study group.
In our study the mean average increase in ACD in Tropicamide group recorded was 0.25 mm from the initial ACD of 3.16 mm (SD: 0.45) (Fig. 1). Limit of agreement was seen in 84.21% of patients (Fig. 2). While in Homatropine group the mean average increase in ACD recorded was 0.29 mm from the initial ACD of 3.09 mm (SD: 0.36 mm) (Fig 3). Limit of agreement was seen in 89.28% of the patients (Fig. 2). The increase in the ACD is due to axial flattening of the lens because of cycloplegia.
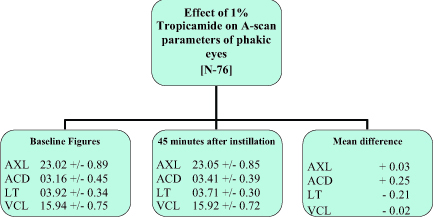
Figure 1: Effect of 1% Tropicamide on A-Scan parameters of phakic eyes
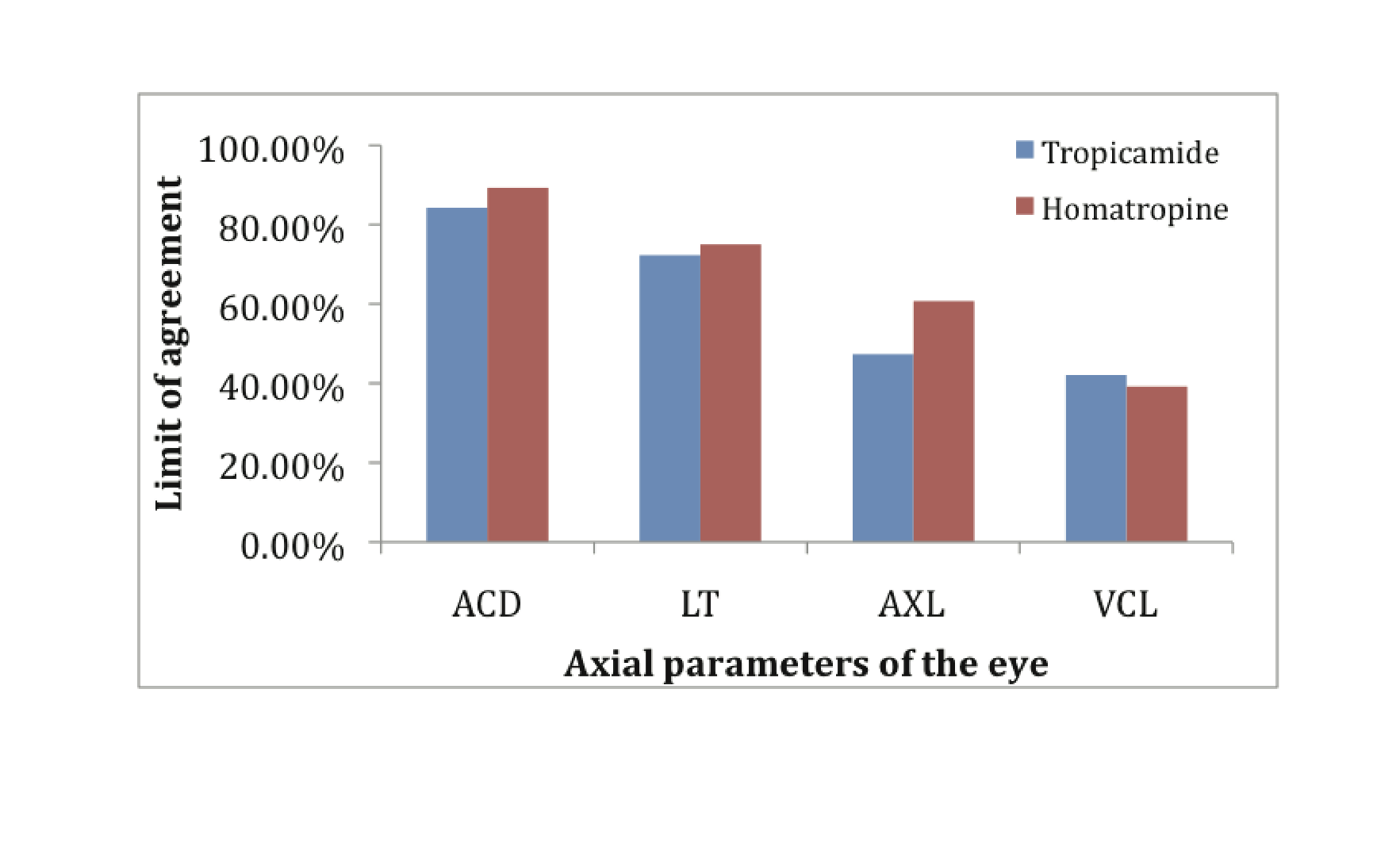
Figure 2: Limit of agreement
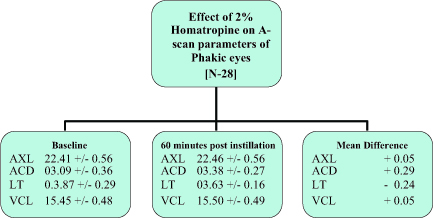
Figure 3: Effect of 2% Homatropine on A-Scan parameters of phakic eyes
Number of patients in percentile shows increase in ACD, decrease in LT, increase in AXL and decrease in VCL in both groups.
The average decrease in the LT in Tropicamide group recorded was 0.21 mm, from the initial LT of 3.92 mm (SD: 0.34) (Fig. 1). Limit of agreement was seen in 72.37% of the patients (Fig. 2). While the average decrease in the LT in Homatropine group recorded was 0.24 mm, from the initial LT of 3.87 mm (SD: 0.29 mm) (Fig. 3). The limit of agreement was seen in 75% of the patients (Fig. 2). The decrease in the LT is due to axial flattening of the lens because of cycloplegia.
There was decrease of 0.02 mm in the VCL in Tropicamide group from the initial of 15.94 mm [SD: 0.74] (Fig. 1). The limit of agreement of decrease in VCL was seen in only 42.11%, while 40.79% showed increase in VCL (Fig. 2). There was 0.05 mm increase in VCL in Homatropine group from the initial VCL of 15.45 mm (SD: 0.48) (Fig. 3). Limit of agreement of increase in VCL was seen only in 46.43% while 39.28% showed decrease in VCL (Fig. 2).
Over all AXL in Tropicamide group increased by 0.03 mm from the initial average AXL of 23.02 mm (SD: 0.89) (Fig. 1).The limit of agreement was seen in only 47.37%, while similar number of patients showed decrease in AXL (Fig. 2). In Homatropine group the overall AXL increased by 0.05 mm from the average baseline readings of 22.41 mm (SD: 0.56) (Fig. 3). The limit of agreement was seen in 60.71% of the patients while 39.28% of the patients in Homatropine group showed decrease in overall AXL (Fig. 2)
Discussion
In this study we measured the axial parameters by A – Scan Ultrasound which is widely available and most commonly used device in general ophthalmic units and represent the "Gold Standard" for biometric dimension.1
Instillation of 1% Tropicamide eye drops and 2% Homatropine eye drops has a deepening effect on anterior chamber depth resulting in increase in anterior chamber depth.2-4
Our results of increase in ACD after using Tropicamide and Homatropine eye drops corresponds with the results of Mehrotra AS 2, Mutti DO 5, Marchini et al 6 and Sheng H 4 who all reported increase in ACD after mydriasis either with Tropicamide or Homatropine eye drops.
Cycloplegia induces decrease in LT due to axial flattening of the lens. We also report decrease in LT after mydriasis with Tropicamide and Homatropine eye drops. Our results of decrease in LT after mydriasis correspond with the results shown by Mutti DO 5 and Marchini.6
The overall AXL increased statistically in both groups, but with lower level of limits of agreements. (Tropicamide group 47.3%, Homatropine group 60.71%)
The VCL increased in Homatropine group [limit of agreement 46.43%] while it is decreased in Tropicamide group by 0.02 mm (limit of agreement 42.11%).
Hence in our study the effect of mydriasis induced by Tropicamide and Homatropine on ACD and LT is statistically conclusive and comparable to other studies with high levels of limit agreement. The effect on overall AXL and VCL is variable and not conclusive due to low levels of limit of agreements.
Conclusion
In the present study 1% Tropicamide and 2% Homatropine eye drops have shown a measurable and conclusive deepening effect on the ACD by 0.25 mm in Tropicamide group and by 0.29 mm in Homatropine group from the initial baseline readings, attributed to axial flattening of the lens because of cycloplegia. Similarly it has got statistically significant effect on LT which decreases on an average by 0.21mm in Tropicamide group and 0.24 mm in Homatropine group. Cycloplegia induced by Tropicamide and Homatropine eye drops had no significant effect on the VCL and the overall AXL of the eye. The reliability of overall AXL and VCL after mydriasis was not conclusive, as the results were variable and limits of agreements were low. As expected, Homatropine has got stronger cycloplegic effect on ACD and LT as compared to Tropicamide, which is reflected statistically on both the parameters.
Acknowledgements
The authors reported no conflict of interest and no funding was received on this work.
|