Type 1 diabetes mellitus (T1DM) is one of the most common chronic diseases in children. Although there are no recent studies documenting T1DM incidence in Omani children, the incidence was earlier documented as 2.45 and 2.62 per 100,000 per year in 1993 and 1994, respectively.1 A higher incidence rate was recently documented in the nearby Gulf countries, as 15.4 per 100,000 per year in Kuwait and 27.5 per 100,000 per year in Saudi Arabia.2,3
Common long-term vascular complications of T1DM include retinopathy, nephropathy, neuropathy, and cardiovascular disease. Although these complications are rare in prepubertal children with T1DM, their prevalence increases during and after puberty.4,5 This can be explained by the fact that the pathogenesis of these vascular complications begins at disease onset. Moreover, Urbina et al,6 described subclinical findings of vascular disease in early childhood. Long-term studies showed delayed onset and slowed progression of microvascular complications with intensive management and good metabolic control,4,7 therefore, optimal glycemic control is an important goal in the management of T1DM. In Oman, there are many factors that affect the care of diabetic patients and result in poor diabetes control, including lack of human resources and a shortage of health professionals specialized in the field of diabetes, partially restricted financial resources for diabetes care, and a lack of updates on treatment protocols. Additionally, patients do not always comply to dietary and therapy advice, and have a poor attitude to exercise.8,9
The Diabetes Control and Complications Trial (DCCT) showed a good relationship between the degree of metabolic control and diabetes complications.7 Intensive management of T1DM can be achieved by using multiple daily injections (MDI) of rapid-acting insulin and a once-daily injection of long-acting insulin, which mimics endogenous insulin secretion characterized by continuous basal insulin secretion and meal-related peaks. Insulin glargine (Lantus; Aventis Pharmaceuticals, US) is a clear basal insulin analogue produced by recombinant DNA technology with duration of action of approximately 24 hours10 and fewer side effects.11
Although many studies have evaluated the use of MDI insulin, there have been no consistent results on the effect on glycemic control.12-20 Several reports showed improvement with this regimen at the onset of diabetes or having switched from a twice daily (BID) to MDI regimen of insulin therapy.12-17 However, some studies revealed that changing to MDI did not have a significant effect, or caused worsening of glycemic control.18-20
To date, there are no previous studies evaluating the use of MDI in children with T1DM from Oman. Although MDI insulin regimen is considered the method of choice in managing T1DM pediatric patients worldwide,21 its implementation in T1DM children in Oman is not yet universal.3 Therefore, this study was conducted to evaluate the effects of switching from a BID regimen to MDI in children and adolescents with T1DM.
Methods
This retrospective cohort study included children and adolescents with T1DM who attended follow-up appointments at the Pediatric Endocrinology Unit, Sultan Qaboos University Hospital (SQUH), Muscat, Oman, between January 2007 and June 2013. All patients with T1DM were identified using the clinic’s records.
The current insulin regimen in all T1DM children and adolescents (<15 years old) was evaluated. Patients were on different insulin regimens including BID insulin, MDI insulin, and insulin pump therapy, as determined by their treating physician and discussed with parents and the patient. The BID regimen consisted of intermediate acting insulin (NPH, or neutral protamine Hagedorn) with short acting insulin (Actrapid; Novo Nordisk) each given once in the morning and once in the evening. The MDI regimen consisted of insulin glargine (Lantus) once daily in the morning with pre-meal short acting insulin given three times daily. For those on MDI, some were started at the time of diagnosis while others were initially on a BID regimen and then switched to MDI based on the decision of the endocrinologist and the parents’ agreement. The latter group was the target of our study in order to assess the effects of this regimen change. Only patients who were receiving BID insulin for at least six months before switching to MDI insulin were included in the study. All children were evaluated every three months at the outpatient clinic. Those with no regular follow-up, a history of non-compliance, and children with less than six months duration from diagnosis of T1DM to time of insulin regimen change were excluded from the study. It is worth mentioning that in those patients who attained puberty, insulin dose was adjusted and increased according to glycemic status.
Clinical information including age, gender, history of hypoglycemic attacks, weight and height measurements, and daily insulin dose per kilogram of body weight were retrieved from the Hospital Information System (HIS). History of hypoglycemic attacks was recorded from clinic visits and relied on history from parents. Examination findings including presence of lipohypertrophy in the patients were documented. Co-existence of autoimmune diseases associated with diabetes such as celiac disease and autoimmune thyroid disease were retrieved from patients’ records. Glycated hemoglobin levels (HbA1c) were measured by high-performance liquid chromatography and reported as HbA1c percentage, according to the National Glycohemoglobin Standardization Program (NGSP). HbA1c levels were monitored routinely every three months in the clinic, and the levels before and after switching insulin from a BID to MDI regimen were obtained. Two HbA1c readings were considered before switching to MDI: one at the time of insulin regimen change, and the other at three months before that, reflecting the six months period prior to insulin regimen change. The mean was calculated for each patient to estimate the baseline HbA1c and used for comparison. Subsequent HbA1c values at three, six, and nine months after changing to MDI were recorded and compared to baseline. A target HbA1c was set as less than 7.5 for all age groups, as recommended by the clinical practice guidelines of the International Society for Pediatric and Adolescent Diabetes (ISPAD).22 The number of patients who had HbA1c of <7.5% was determined and compared between the baseline and after switching to MDI. Body mass index (BMI) was calculated (weight(kg)/height(m2)) for each patient before MDI change and nine months after. BMI z-scores were obtained for each patient according to World Health Organization growth standards.23
Ethical approval for this study was obtained from the Research and Ethics Committees, College of Medicine and Health Sciences, Sultan Qaboos University, Muscat, Oman.
The data was analyzed using SPSS, version 20. The number (percentage) and mean±standard deviation (SD) were calculated for demographic data, and for HbA1c values before and after insulin regimen change. The differences between categorical variables before and after switching to MDI regimen were assessed by chi-square test or Fisher’s exact test as appropriate. Comparing the mean±SD before and after switching BID to MDI regimen was done using the paired Student’s t-test. A probability (p) value of <0.050 was used as a cut-off for all tests of statistical significance.
Results
Among 146 patients with T1DM followed-up at Pediatric Endocrinology Unit during the six-year study period, 12 (8%) were using insulin pump therapy, 23 (16%) were on BID insulin, and 109 (76%) were using MDI. Among the 109 children who were using a MDI insulin regimen, 53 (49%) were initially on BID insulin and then switched to MDI regimen and, therefore, evaluated for inclusion in the study [Figure 1]. The other 56 (51%) children were either started on this regimen since the time of diagnosis or were on MDI regime for less than six months. Three patients were switched within three to six months after diagnosis and were excluded from the study. In addition, six patients were lost to follow-up, and one patient had a significant history of non-compliance and was excluded from the study. The remaining 43 patients were 58% (25) male and 42% (18) female, with a mean age of 9.4±3.7 years at time of insulin regimen change [Figure 1]. There was no documented lipohypertrophy encountered in our studied patients. Three patients were diagnosed as celiac disease and were already on treatment (a gluten free diet) before the study. No cases with autoimmune thyroid diseases were found among our cohort.
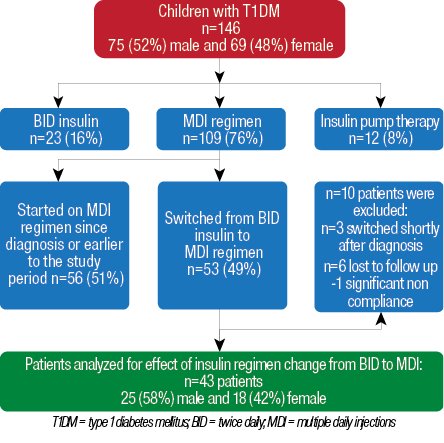
Figure 1: Flowchart and demographics of patients with T1DM followed-up at the Pediatric Endocrinology Unit at Sultan Qaboos University Hospital for evaluation of insulin regimen change from twice daily to multiple daily injections.
The effects of switching insulin regimen from BID to MDI are shown in Table 1. There was a significant decrease in mean HbA1c values (reported as percentage) from baseline compared to three months after switching from BID to MDI, with mean±SD of 10.0±1.6 and 9.5±1.6, respectively (p=0.023). The mean±SD for calculated percentage of improvement in HbA1c at three months follow-up was 4.4±14. Nevertheless, this improvement was less significant in the subsequent follow up visits at six and nine months [Figure 2]. Compared to a mean±SD baseline HbA1c of 10.0±1.6 while on BID regimen, the mean±SD HbA1c was 9.58±1.8 at six months after MDI (p=0.100) and 9.61±2.0 at nine months after MDI (p=0.134). One patient (2%) met the HbA1c therapeutic target of <7.5 while on a BID regimen, which increased to 4 patients (9%) at three months, five patients (12%) at six months, and 7 patients (16%) at nine months after switching to MDI. However, this change was not statistically significant (p>0.050).
Table 1: The effect of switching insulin regimen from twice daily (BID) to multiple daily injections (MDI) on HbA1c (reported in percentage) at three, six, and nine months (n=43).
|
HbA1c*,** |
10.0±1.6 |
9.50±1.6 |
9.58±1.8 |
9.61±2.0 |
|
Change in HbA1c** |
|
-0.52±1.44 |
-0.44±1.70 |
- 0.41±1.80 |
|
Calculated percentage change in HbA1c** |
|
- 4.4±14 |
-3.4±17 |
-3.5±16 |
|
p-value † |
|
0.023 |
0.100 |
0.134 |
|
Patients with HbA1c <7.5*** |
1(2) |
4(9) |
5(12) |
7(16) |
* HbA1c expressed in percent, according to the National Glycohemoglobin Standardization Program; ** mean±SD; ***n(%)
† p-value using paired t-test, comparing mean HbA1c at baseline and follow-up at three, six, and nine months after switching to MDI.
‡ p-value using Fisher’s exact test, comparing the number of patients with HbA1c <7.5 at baseline and at three, six, and nine months after switching to MDI.
§ Baseline is considered as the mean of the two latest HbA1c readings before changing to MDI insulin regimen.
SD = standard deviation.
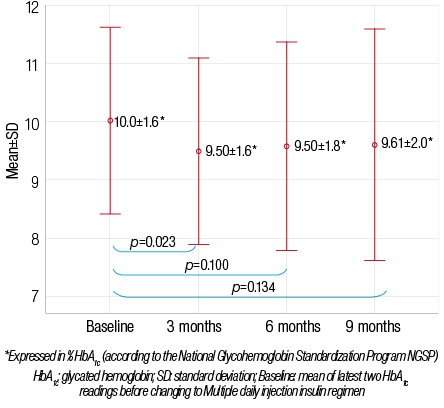
Figure 2: Changes in mean HbA1c before and after switching to multiple daily injections (n=43).
On the other hand, there was an overall decrease in mean daily insulin dose, with a mean±SD total insulin (expressed as IU per kilogram of body weight per day) of 1.22±0.43 during BID insulin regimen, and 1.11±0.25 when switching to MDI (p=0.048). In addition, switching regimens did not worsen the occurrence of hypoglycemia, but, rather, there was an observed improvement. Twenty-three percent (n=10) patients reported episodes of hypoglycemia while on the BID regimen and 11% (n=5) after switching to MDI (p=0.154). There was no significant difference in the mean BMI z-scores before and after switching from BID to MDI (0.12±1.21 and 0.14±1.18, respectively;
p=0.838) [Figure 3].
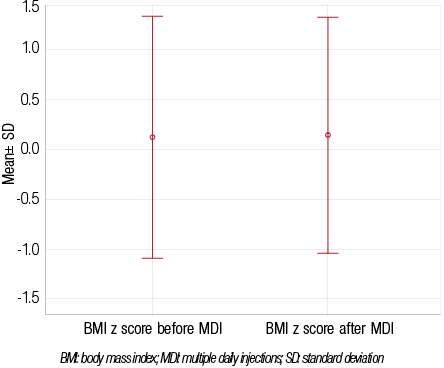
Figure 3: Changes in mean BMI z-scores before and after switching to multiple daily injections (n=43).
The HbA1c response to switching from BID to MDI for different age groups is illustrated in Table 2. In children aged less than five years (n=5), the mean HbA1c initially increased at three months after the regimen change, but then started dropping in the subsequent follow-ups at six and nine months; however, this was not statistically significant. For children aged five to 11 years (n=21), there was a significant decrease in HbA1c at three months after switching to MDI regimen. Again, this improvement was less significant thereafter. In those aged greater than 11 years (n=17) there was some improvement in HbA1c but the difference was not statistically significant at any of the follow-up time points.
Table 2: Effects of switching twice daily (BID) to multiple daily injections (MDI) insulin regimen according to age at various time points.
* HbA1c expressed in percent, according to the National Glycohemoglobin Standardization Program.
** mean±SD
† Baseline is considered as mean of two latest HbA1c readings before switching to MDI.
‡ using paired t-test, comparing the mean HbA1c at baseline and at three, six, and nine months after switching to MDI.
Discussion
Our study reports the effect on the metabolic and glycemic control of T1DM children and adolescents when switching from regular BID insulin regimen to intensive insulin therapy with MDI, from a single center in Oman. While MDI insulin is considered the method of choice in managing T1DM pediatric patients worldwide,21 use of this regimen is not yet universal to T1DM children in Oman and many other countries.3 This is the first report on the metabolic control of children using a MDI insulin regimen in Oman.
Although our cohort had poor glycemic control to begin with (HbA1c=10±1.6), the overall control improved significantly at three months after switching from BID to MDI insulin. However, this improvement was less significant in the subsequent follow-ups at six and nine months. One possible explanation is a reduction in motivation, which was initially present after initiating the new treatment regimen, and was followed by reduced compliance afterwards reflected as less significant improvement in the mean HbA1c at these two time points.
Improved glycemic control using intensive insulin therapy compared to conventional twice-daily insulin therapy is well documented by numerous reports.12-17 Controlled studies from the Western countries demonstrated significantly lower mean daily blood glucose levels12 and significantly decreased median HbA1c levels.13 In addition, observational studies have also reported improved glycemic control in children and adolescents, aged between 2 to 19 years, using intensive insulin regimen compared to conventional therapy.14-16 Another observational study from Egypt reported that a MDI regimen was associated with a greater frequency of good glycemic control.17
Nevertheless, few studies showed that a change to MDI regimen does not have a significant effect, or cause worsening in glycemic control when MDI delivery of insulin was implemented.18,19 In addition, a study from Saudi Arabia revealed a higher percentage of subjects receiving intensive insulin therapy were poorly controlled. However, the study cohort consisted mainly of patients on intensive insulin therapy (89.7%) which precluded precise comparisons between conventionally and intensively treated patients.20
With regards to the improvement in glycemic control after switching BID to MDI insulin in different age groups, those aged 5–11 years old had the best outcome among all groups, with a significant improvement in HbA1c at three months. However, the improvement in the subsequent follow-ups at six and nine months was not statistically significant. Schober et al,11 found no significant difference in HbA1c levels in the same age group between the BID versus MDI insulin use. On the other hand, HbA1c levels did not improve in the toddlers and children (<5 years old) after changing BID to MDI insulin in our study, but the number of patients was only five and, therefore, it is difficult to draw a reliable conclusion from our patients in this age group. In addition, this observation in patients under the age of five could be explained by frequent snacking or the possibility that the parents were administering a lower dose due to fear of hypoglycemia. This lack of metabolic improvement in intensively treated young infants and toddlers was also observed by Tonella et al,24 Dixon et al,25 and Dorchy et al.26 However, a significant decrease in HbA1c in preschool children was reported in other studies.27,28 Although some improvement was noted in the HbA1c level of our adolescent (age >11 years) patients after switching to MDI, this did not reach statistical significance. This poor control observed in our study might be secondary to poor compliance to insulin therapy and dietary advice, as well as lack of supervision by parents while the child administered their own insulin injections. Similar poor metabolic control was observed in adolescents after switching to MDI in other studies.19,29 On the other hand, adolescents in the DCCT and other trials showed improved metabolic control with MDI insulin.7 This may be due to discrepancies in the study type, sample size, and patient characteristics in the different studies.
We observed a less frequent history of hypoglycemia attacks in MDI insulin regimen compared to conventional BID insulin therapy, although this was not statistically significant. However, the history of hypoglycemia was only retrieved from patient notes and was not directly observed from blood sugar monitoring. The finding of more stable glycemic control and fewer hypoglycemia attacks using MDI has been reported by other studies.12,14,15 This can be explained by the fact that glargine insulin shows a flat profile of plasma insulin levels, and no pronounced peak of activity, associated with a lower relative risk of hypoglycemia.27 Nevertheless, both the DCCT7 and Jackson et al,30 reported that good metabolic control results in unavoidable hypoglycemia. In this study, and comparable to other reports including the study by Colino et al,28 we found significantly less daily insulin requirement. In addition, the BMI did not increase in any of the age groups, which could be due to the reduction of symptomatic hypoglycaemia, less usage of extra carbohydrates, and less overall insulin dose. This finding was reported by Colino et al28 as well as Päivärinta et al.29
Despite all the aforementioned benefits of the MDI insulin regimen, there is a need for increasing blood glucose monitoring and the frequency of insulin administration. Therefore, it is crucial to have proper patient/family education in order to achieve better outcome. It is worth noting that patients on a MDI regimen were measuring their blood glucose level around four times per day, but this was not directly analyzed in our study because of a lack of availability of full records due to retrospective nature of the study.
The main limitation to our findings is that this is a retrospective, non-randomized, uncontrolled study. In addition, direct evaluation of compliance was not possible, which might have affected the metabolic control being assessed after starting the MDI regime. Although dietary habits and daily carbohydrate consumption is an important consideration, with respect to the effect of insulin regimen change, this was not directly compared in our study.
Conclusion
In conclusion, intensive insulin therapy using a MDI regimen has favorable effects on the overall control of children and adolescents with T1DM compared to a BID regimen. The MDI insulin regimen requires more cooperation and the understanding of both the patients and their families towards this commitment of increased insulin injections as well as the frequency of blood glucose monitoring, in order to show more beneficial effects. In our study, MDI also resulted in a reduced total daily insulin dose, without affecting BMI. In addition, patients had fewer attacks of hypoglycemia after changing to MDI, although this was not statistically significant. However, due to small number of patients included in the study, the results may not be generalizable to the entire population. A larger multicenter study is recommended to reflect the effect of this therapeutic option and reveal the associated factors involved in the response of the Omani population compared to other countries.
Disclosure
The authors reported no conflict of interests. No funding was received for this work.
references
- Soliman AT, al-Salmi IS, Asfour MG. Epidemiology of childhood insulin-dependent diabetes mellitus in the Sultanate of Oman. Diabet Med 1996 Jun;13(6):582-586.
- Shaltout AA, Qabazard MA, Abdella NA, LaPorte RE, al Arouj M, Ben Nekhi A, et al; Kuwait Study Group of Diabetes in Childhood. High incidence of childhood-onset IDDM in Kuwait. Diabetes Care 1995 Jul;18(7):923-927.
- Abduljabbar MA, Aljubeh JM, Amalraj A, Cherian MP. Incidence trends of childhood type 1 diabetes in eastern Saudi Arabia. Saudi Med J 2010 Apr;31(4):413-418.
- Vasudevan AR, Burns A, Fonseca VA. The effectiveness of intensive glycemic control for the prevention of vascular complications in diabetes mellitus. Treat Endocrinol 2006;5(5):273-286.
- Al-Sinani S, Sharef SW, Al-Yaarubi S, Al-Zakwani I, Al-Naamani K, Al-Hajri A, et al. Prevalence of celiac disease in omani children with type 1 diabetes mellitus: a cross sectional study. Oman Med J 2013. Jul;28(4):260-263.
- Urbina EM, Wadwa RP, Davis C, Snively BM, Dolan LM, Daniels SR, et al. Prevalence of increased arterial stiffness in children with type 1 diabetes mellitus differs by measurement site and sex: the SEARCH for Diabetes in Youth Study. J Pediatr 2010 May;156(5):731-737, e1.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993 Sep;329(14):977-986.
- Al-Yaarubi S, Ullah I, Sharef SW, Al Shidhani, Al Hanai, Al Kalbani, Al Jamoodi S. Demographic and Clinical Characteristics of Type 1 Diabetes Mellitus in Omani Children-Single Center Experience.Oman Med J. 2014 Mar; 29(2): 119–122.
- Al-Yaarubi S. Diabetes Care in Oman: Obstacles and solutions. Sultan Qaboos Univ Med J 2011 Aug;11(3):343-348.
- Rosenstock J, Park G, Zimmerman J; U.S. Insulin Glargine (HOE 901) Type 1 Diabetes Investigator Group. Basal insulin glargine (HOE 901) versus NPH insulin in patients with type 1 diabetes on multiple daily insulin regimens. U.S. Insulin Glargine (HOE 901) Type 1 Diabetes Investigator Group. Diabetes Care 2000 Aug;23(8):1137-1142.
- Schober E, Schoenle E, Van Dyk J, Wernicke-Panten K; Pediatric Study Group of Insulin Glargine. Comparative trial between insulin glargine and NPH insulin in children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2002 Apr;15(4):369-376.
- Murphy NP, Keane SM, Ong KK, Ford-Adams M, Edge JA, Acerini CL, et al. Randomized cross-over trial of insulin glargine plus lispro or NPH insulin plus regular human insulin in adolescents with type 1 diabetes on intensive insulin regimens. Diabetes Care 2003 Mar;26(3):799-804.
- Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994 Aug;125(2):177-188.
- Chase HP, Dixon B, Pearson J, Fiallo-Scharer R, Walravens P, Klingensmith G, et al. Reduced hypoglycemic episodes and improved glycemic control in children with type 1 diabetes using insulin glargine and neutral protamine Hagedorn insulin. J Pediatr 2003 Dec;143(6):737-740.
- Hathout EH, Fujishige L, Geach J, Ischandar M, Maruo S, Mace JW. Effect of therapy with insulin glargine (lantus) on glycemic control in toddlers, children, and adolescents with diabetes. Diabetes Technol Ther 2003;5(5):801-806.
- Alemzadeh R, Ellis JN, Holzum MK, Parton EA, Wyatt DT. Beneficial effects of continuous subcutaneous insulin infusion and flexible multiple daily insulin regimen using insulin glargine in type 1 diabetes. Pediatrics 2004 Jul;114(1):e91-e95.
- Mohammad HA, Farghaly HS, Metwalley KA, Monazea EM, Abd El-Hafeez HA. Predictors of glycemic control in children with Type 1 diabetes mellitus in Assiut-Egypt. Indian J Endocrinol Metab 2012 Sep;16(5):796-802.
- Beck JK, Lewis TV, Logan KJ, Harrison DL, Gardner AW, Copeland KC. Intensive vs. conventional insulin management initiated at diagnosis in children with diabetes: should payer source influence the choice of therapy? Pediatr Diabetes 2009 Sep;10(6):368-373.
- Flück CE, Kuhlmann BV, Mullis PE. [Metabolic control in children and adolescents with diabetes mellitus type I in Berne: a cross-sectional study]. Schweiz Med Wochenschr 1999 Nov;129(44):1650-1655.
- Al-Agha A, Ocheltree A, Hakeem A. Metabolic control in children and adolescents with insulin-dependent diabetes mellitus at King Abdul-Aziz University Hospital. J Clin Res Pediatr Endocrinol 2011;3(4):202-207.
- Mühlhauser I, Bruckner I, Berger M, Cheţa D, Jörgens V, Ionescu-Tîrgovişte C, et al. Evaluation of an intensified insulin treatment and teaching programme as routine management of type 1 (insulin-dependent) diabetes. The Bucharest-Düsseldorf Study. Diabetologia 1987 Sep;30(9):681-690.
- Hanas R, Donaghue KC, Klingensmith G, Swift PG. ISPAD clinical practice consensus guidelines 2009 compendium. Introduction. Pediatr Diabetes 2009 Sep;10(Suppl 12):1-2.
- de Onis M, Onyango A, Borghi E, Siyam A, Blössner M, Lutter C; WHO Multicentre Growth Reference Study Group. Worldwide implementation of the WHO Child Growth Standards. Public Health Nutr 2012 Sep;15(9):1603-1610.
- Tonella P, Flück CE, Mullis PE. Metabolic control of type 1 diabetic patients followed at the University Children’s Hospital in Berne: have we reached the goal? Swiss Med Wkly 2010;140:w13057.
- Dixon B, Peter Chase H, Burdick J, Fiallo-Scharer R, Walravens P, Klingensmith G, et al. Use of insulin glargine in children under age 6 with type 1 diabetes. Pediatr Diabetes 2005 Sep;6(3):150-154.
- Dorchy H, Roggemans MP, Willems D. Glycated hemoglobin and related factors in diabetic children and adolescents under 18 years of age: a Belgian experience. Diabetes Care 1997 Jan;20(1):2-6.
- Alemzadeh R, Berhe T, Wyatt DT. Flexible insulin therapy with glargine insulin improved glycemic control and reduced severe hypoglycemia among preschool-aged children with type 1 diabetes mellitus. Pediatrics 2005 May;115(5):1320-1324.
- Colino E, López-Capapé M, Golmayo L, Alvarez MA, Alonso M, Barrio R. Therapy with insulin glargine (Lantus) in toddlers, children and adolescents with type 1 diabetes. Diabetes Res Clin Pract 2005 Oct;70(1):1-7.
- Päivärinta M, Tapanainen P, Veijola R. Basal insulin switch from NPH to glargine in children and adolescents with type 1 diabetes. Pediatr Diabetes 2008 Jun;9(3 Pt 2):83-90.
- Jackson A, Ternand C, Brunzell C, Kleinschmidt T, Dew D, Milla C, et al. Insulin glargine improves hemoglobin A1c in children and adolescents with poorly controlled type 1 diabetes. Pediatr Diabetes 2003 Jun;4(2):64-69.