|
Introduction
Islet cell tumors are rare pancreatic or peripancreatic tumors which constitute a broad spectrum of endocrine neoplasms. They are classified on clinical grounds by the symptoms produced from hormone production. Functioning islet cell tumors (clinically evident or syndromic islet cell tumors) produce symptoms related to excessive hormone production and thus are typically diagnosed early when the lesions are smaller than 2 cm.1 On the contrary, non-functioning islet cell tumors (clinically silent or nonsyndromic islet cell tumors) do not produced symptoms related to excessive hormone production, although these tumors are still hormonally active.2,3
Nonfunctional islet cell tumors typically represent one half of all islet cell tumors and are usually greater than 5 cm at the time of diagnosis.2 Clinically, nonfunctioning islet cell tumors affect patients from 24-74 years with a mean age of 57. Patients are mostly asymptomatic, but when symptomatic, they present with abdominal pain, jaundice, variceal bleeding, palpable mass and gastric outlet obstruction.4
Nonfunctioning tumors are usually located in the pancreatic body and tail. 5 These tumors are often diagnosed at advanced stages of the disease and produce symptoms due to mass effect from their large size. As imaging technology advances, smaller non-functioning islet cell tumors are being diagnosed in asymptomatic patients.6
Histologically, functioning and nonfunctioning islet cell tumors are identical with three observed patterns: (1) solid, diffuse pattern; (2) ribbon like, trabecular pattern; and (3) an acinar or duct like pattern. In addition, immunohistochemical staining does not allow reliable differentiation between functioning and nonfunctioning islet cell tumors.3
Imaging features of pancreatic NFICT can overlap with other pancreatic masses. Given that the prognosis and treatment is different. It is very important to be able to diagnose this rare pancreatic neoplasm. We therefore describe the cross sectional imaging features of 28 patients with proven histological diagnosis of NFICT.
Methods
Approval was obtained from the of Institutional Board Review at BJ Hospital, Mallinkrodt Institute of Radiology., Saint Louis Missouri, USA Computed tomogram of 28 patients (15 male,13 female)with pathologically proven diagnosis of Non-Functional Islet cell tumor of the pancreas performed at our institution between March 2002-July 2006, were retrospectively analyzed by two abdominal radiologists in consensus with regard to size, site of pancreatic origin, calcification, pattern of enhancement, local or vascular invasion and distant Metastases; size, number, pattern of enhancement, vascular invasion, and biliary ductal dilatation.
The patients Ages ranged between 22-84 years old. The method of obtaining histological diagnosis included open surgical in 10 patients, image guided percutaneous biopsy in 15 patients, and endoscopic ultrasound biopsy in 3 patients. Imaging indication in these patients included: abdominal pain in 13 patients, abdominal mass, pain and weight loss in 12 patients, Jaundice in 2 patients and Incidental diagnosis in one patient.
All CT examinations were performed with either 16 or 64 slice Siemens Scanners. Patients received 125 ml of Optiray 350 which was administered at a rate of 4 ml/sec with a power injector. 750 ml of water was administered 45 minutes prior to the study in order to achieve good distention of stomach and duodenum. An unenhanced scan was obtained initially followed by images acquired during both arterial and venous phase.
Results
Pancreatic NFICT size ranged from 1.2-13 cm. The patterns of enhancement were: hypervascular homogenous 4, hypervascular heterogenous in 24 cases (Table 1). 5 of the pancreatic NFICT cases had upstream dilatation of both common bile duct (CBD) and pancreatic duct (PD), 4 with only PD dilatation. The invasion of nearby local structures were: spleen 3, stomach 1, spleen, stomach and adrenal in 1 case.
Table 1: Pancreatic NFICT pattern of enhancement
|
Pattern of enhancement
|
Number of cases (%)
|
|
Hypervascular homogenous
|
4 (14%)
|
|
Hypervascular heterogenous
|
24 (86%)
|
|
Hypodense
|
|
| Total No of cases |
28 |
The remaining 23 cases had no local invasion 18 of the 28 cases had invasion of nearby local vessels, while the remaining 10 did not. Metastatic liver lesions in the 19 cases ranged in size from 1-8 cm.
Patterns of liver lesion enhancement were: hypervascular homogenous 4, hypervascular heterogenous with peripheral enhancement and central necrosis 15.5 of the 19 cases with hepatic metastases were associated with invasion/encasement of intrahepatic vessels.
The common imaging features of the hepatic metastases were: Medium to large size (3-8 cm) lesions ranging in number from single to multiple with heterogenous peripheral enhancement and central necrosis found in 8 cases, small(1-2 cm) innumerable peripherally enhancing lesions with central necrosis giving rise to a pseudo-cirrhosis appearance found in 6 cases, small and few with solid homogenous enhancement in 4, small(1-2 cm) multiple with peripheral enhancement and central necrosis in 1. (Table 2)
Table 2: Metastatic liver lesions: Imaging features.
|
Imaging features
|
Number ofcases (%)
|
|
Medium to large size multiple heterogenous enhancing with central necrosis
|
8 (42%)
|
|
Small innumerable peripheral enhancing lesions with central necrosis (psudo-cirhosis)
|
6 (32%)
|
|
Small multiple solid homogenous enhancing lesions
|
4 (21%)
|
|
Small few peripheral enhancing lesions with central necrosis
|
1 (5%)
|
| Total No of cases |
19 |
Discussion
The results of the study shows that the majority of functional islet cell tumors are usually less than 3 cm in size at the time of diagnosis, and are typically homogenous hyperenhancing lesions and best seen during arterial phase.2
In contrast cystic degeneration, calcification and necrosis are more common in larger syndromic islet cell tumors such as Glucagonomas, Vipomas and nonfunctional islet cell tumors.2 Insulinoma is the common functional islet cell tumor and is usually single and intrapancreatic in location.2 In contrast gastrinomas are usually multiple and extrapancreatic in location.2
The computed tomographic appearance of nonfunctioning islet cell tumors typically includes: diffuse solid homogenous enhancement which tends to be iso to hyperdense relative to the normal pancreatic parenchyma (fig. 1). Hypervascular heterogenous enhancement with areas of necrosis and cystic degeneration (fig. 2). Hypodense appearing similar to ductal carcinoma.5-7
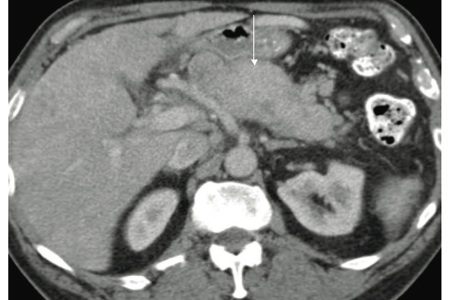
Figure 1: Pancreatic NFICT. Contrast enhanced CT shows a diffuse hypervascular homogenously enhancing mass involving most of the pancreas (white arrow).
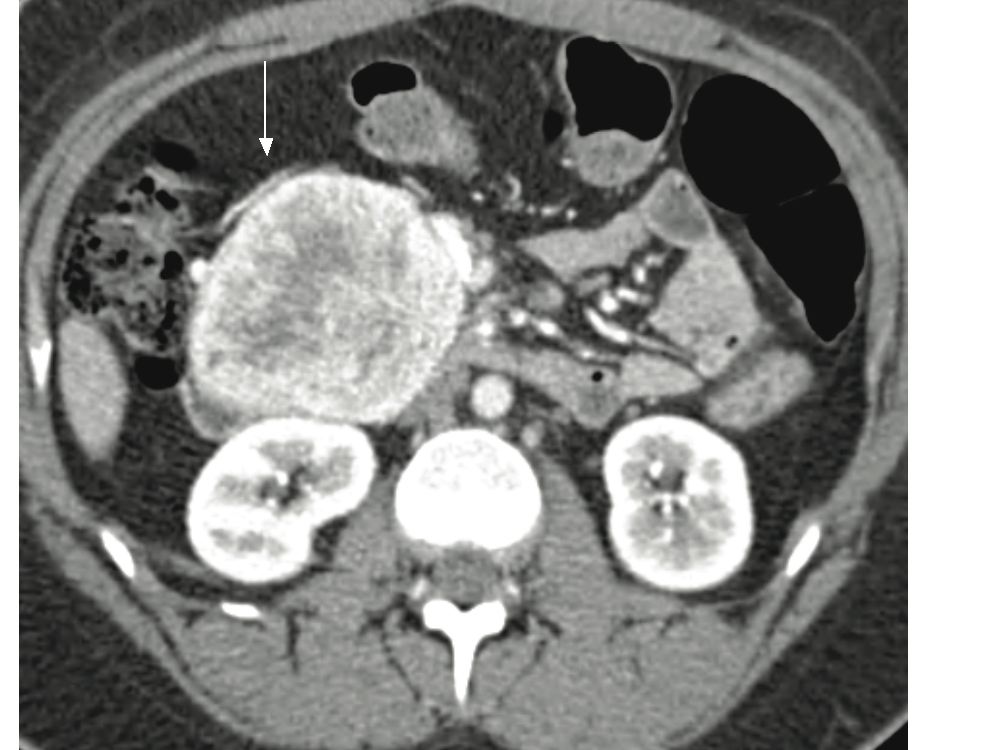
Figure 2: Pancreatic NFICT. Contrast enhanced CT demonstrates a circumscribed heterogenously enhancing mass in the pancreas (white arrow).
In our series the commonest imaging pattern was hypervascular heterogenous enhancement which is similar to what was reported in previous series.5-7 In our study coarse nodular calcification was present in up to 20% of cases which is consistent with previous data.5 Local invasion, vascular invasion and metastatic disease are more commonly seen with the larger tumors.
Metastatic regional lymphadenopathy is seen in about 37%, while dilatation of pancreatic and common bile duct is present in 15% and 19% of patients with NFICT respectively. 5 Venous involvement by large NFICT is common with the three type of venous abnormalities being: venous occlusion, venous encasement, and intravenous tumor extension. 8 (fig. 3). The commonest venous involvement we encountered was venous occlusion and encasement. Celiac axis and superior mesenteric artery are rarely involved.5 Hepatic metastases are seen in 57% of these patients.5 Liver involvement is considered a major prognostic factor for survival.9 At CT, they typically demonstrate homogenous enhancement, peripheral enhancement with cental low density, and uniformly low density.5 (fig. 4,5).
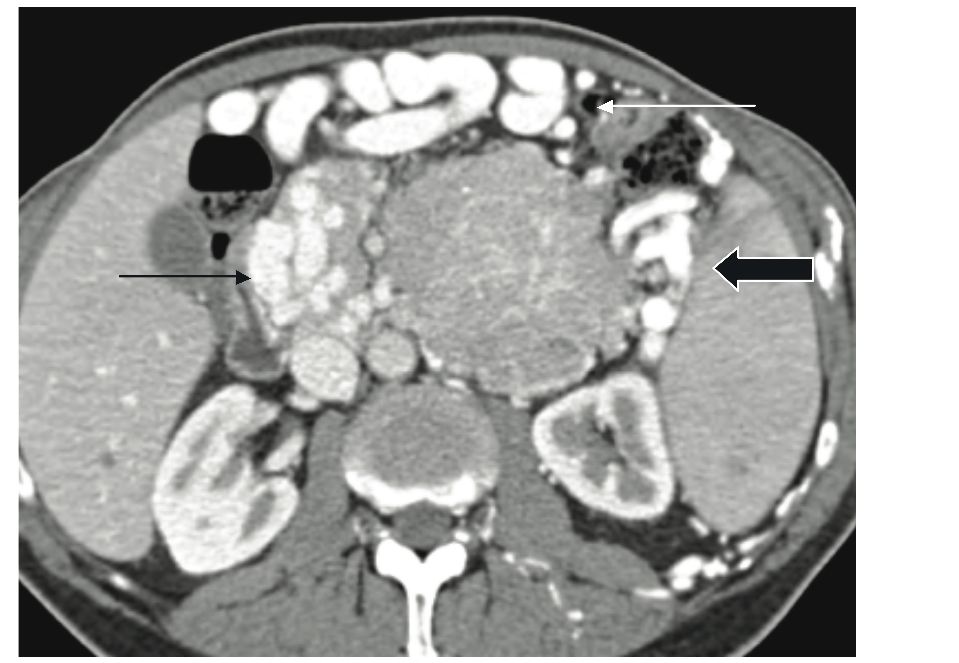
Figure 3: Patient with pancreatic NFICT. Large pancreatic heterogenously enhancing mass with superior mesenteric and splenic vein invasion (not shown) resulting in perisplenic (Thick black arrow), gastroepiploic (white arrow) and pancreaticoduodenal varices (Black arrow).
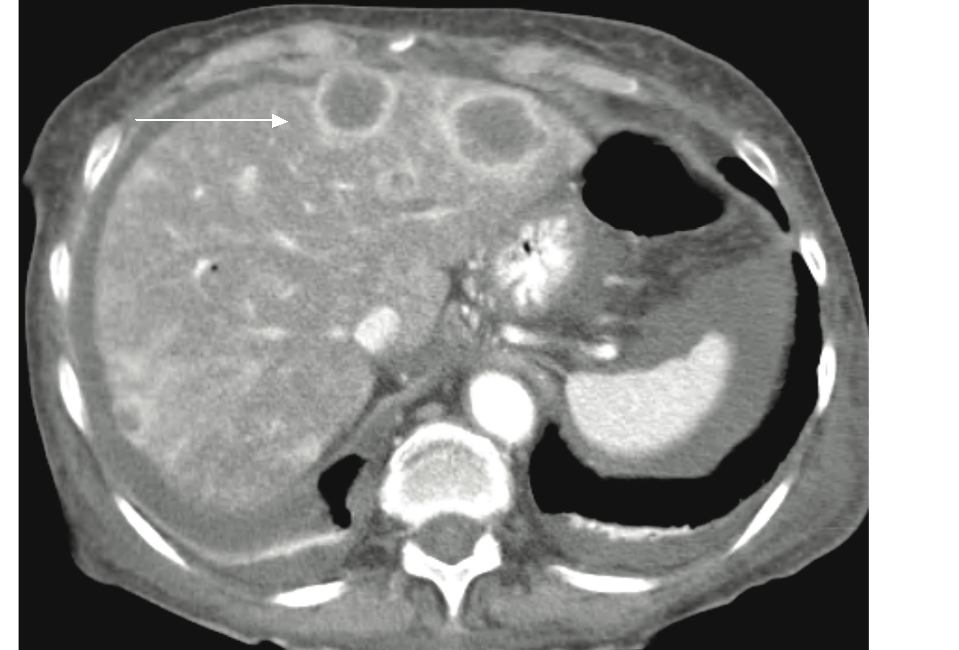
Figure 4: Patient with pancreatic NFICT and hepatic metasteses. Two liver metastatic peripheral enhancing lesions with central area of necrosis (white arrow).
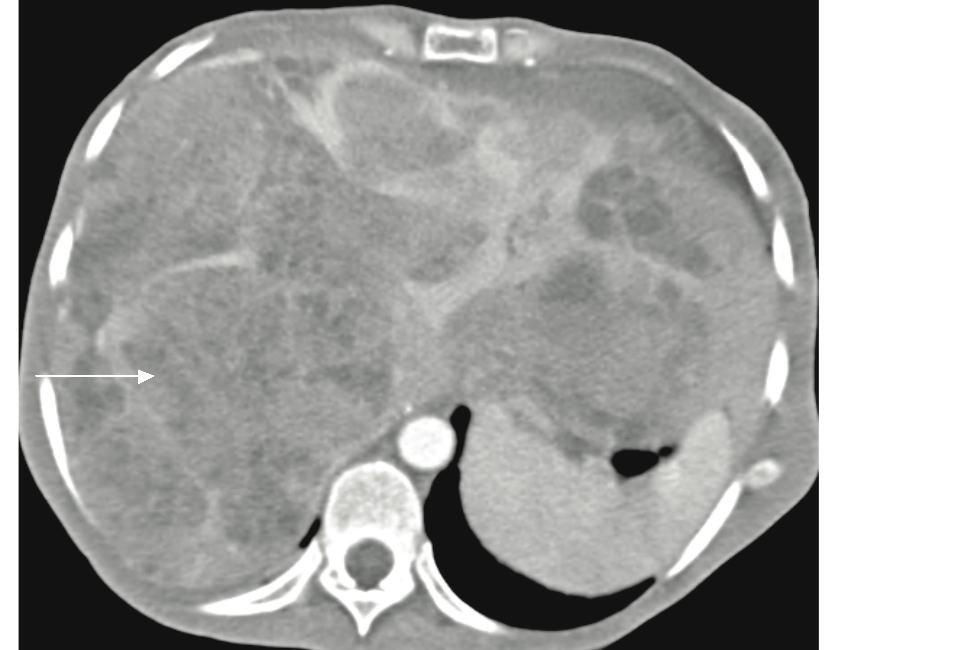
Figure 5: Patient with pancreatic NFICT (not shown) with diffuse multiple hepatic metastases. There are multiple large heterogenously enhancing lesions in the liver with areas of necrosis (white arrow).
In our series the commonest metastatic liver lesion imaging pattern was hypervascular heterogenous enhancment with areas of low density indicative of necrosis. We also observed a pattern of innumerable diffuse small metastatic liver lesions with mixed hypodense and heterogenous enhancement resulting in pseudo-cirrhosis appearance (fig. 6). Pancreatic ductal adenocarcinoma is the commonest malignant pancreatic neoplasm accounting for approximately 85% of pancreatic neoplasms. They usually occur in patients in the seventh decade with a distinct male predominance. At CT, they typically appear as low attenuation masses that invade adjacent vessels.10 Lymph nodes metastases are frequently present. Solid Papillary epithelial neoplasm occur almost exclusively in women, the majority of whom are in the second and third decades. At imaging, they appear as well defined solid masses with variable degrees of cystic degeneration.11 Peripheral calcification are seen in a third of tumors.
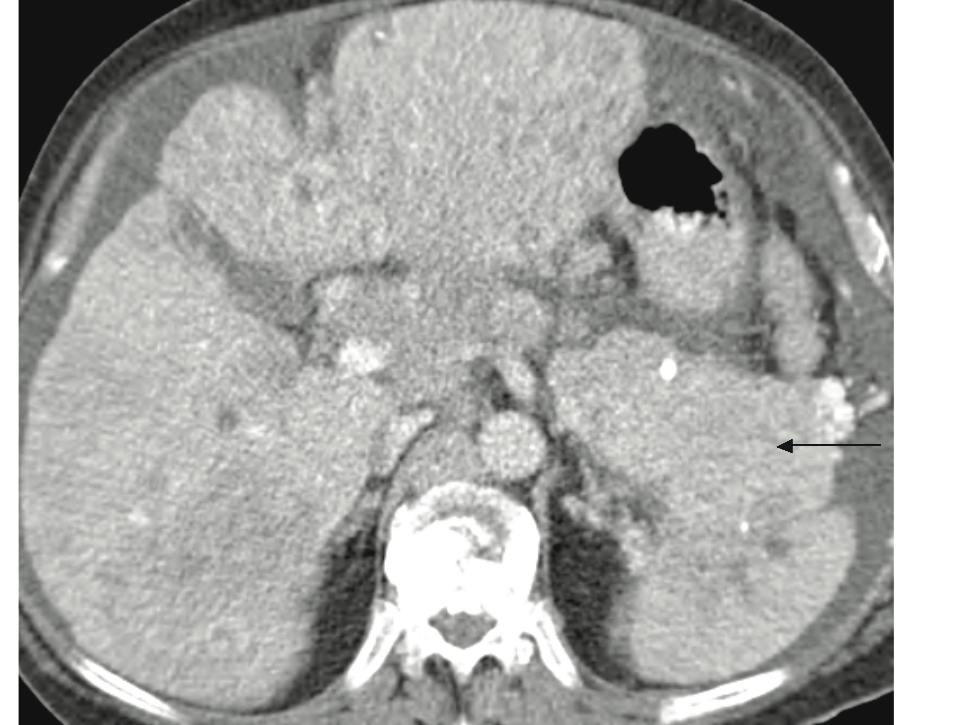
Figure 6: Patient with pancreatic NFICT and multiple small hepatic metastases. There is a heterogenously enhancing mass in pancreatic body and tail (Black arrow). There are multiple diffuse small hypodense and heterogenously enhancing lesions throughout the liver resulting in pseudocirrhosis appearance.
Pancreatic mucinous cystic tumors occur almost exclusively in women in fifth or sixth decade. Most of these tumors are symptomatic at presentation and occur more frequently in the body and tail of the pancreas. On imaging, tumors commonly occur as unilocular or multilocular macrocystic lesions that may have mural nodules with peripheral rim calcification being seen in up to 10% of patients.11
Conclusion
The differential diagnosis of islet cell tumors commonly includes pancreatic adenocarcinoma and solid papillary epithelial neoplasm, and to a lesser extent a mucinous cystadenocarcinoma. Accurate diagnosis of the specific type of pancreatic tumor is important in terms of prognosis and management. Due to overlapping imaging features, combining the imaging features of pancreatic NFICT and its hepatic metastases will aid in narrowing the differential diagnosis. An FNA or excisional biopsy is often required for histological diagnosis.
Acknowledgements
The authors reported no conflict of interest and no funding was received on this work.
|