|
Abstract
Traumatic rupture of the thoracic aorta is a leading cause of death, following major blunt trauma, and endovascular repair has evolved as a viable alternative to open repair. This report highlights the role of transesophageal echocardiography as a valuable imaging tool for locating the exact position of the lesion, guiding placement of the endograft, detecting leaks around it and supplementing information derived from angiography during endograft deployment.
Keywords: Aorta; Thoracic injuries; Echocardiography transesophageal; Blood Vessel Prosthesis Implantation; Stents..
Introduction
Endovascular repair of traumatic thoracic aortic transection has emerged as a viable alternative to surgical management.1 Transesophageal echocardiography (TEE) is recommended as an adjunctive imaging technique during Thoracic Endovascular Aortic Repair.2 Steep left anterior oblique view is usually used during aortic angiography to identify abnormalities in the aortic arch and proximal descending aorta. In case of traumatic aortic transection, two dimensional left anterior oblique view can identify the level of the lesion, but needs additional views with more contrast administration to ascertain the exact site of the lesion. This case report highlights the role of TEE in localizing the exact site of aortic partial transection, which was at the isthmus in the posterior wall of the aorta very close to the left subclavian artery origin that was difficult to image by standard views of aortography. The endograft could be deployed safely and accurately without compromising the left subclavian artery origin under TEE guidance.
Case Report
A 40-years-old man sustained a decelerating thoracic aortic injury following a motor vehicle collision. He was brought to the accident and emergency room in a hemodynamically and neurologically stable condition. He was a known hypertensive on treatment (Tab. Lisinopril 10 mg once a day). Computerized tomography (CT) of brain and spine displayed no signs of injury. CT of the chest showed partial transection of the aorta involving descending aorta just distal to the origin of left subclavian artery with a proximal intimal flap and a pseudoaneurysm. A left hemothorax was also present. After obtaining an informed consent, an emergency endovascular stent repair was planned.
The procedure was performed in the cardiac catheterization laboratory under general anesthesia and standard American Society of Anesthesiologists (ASA) monitoring. Invasive monitoring involved cannulation of the left radial artery and right internal jugular vein. Surgical access was achieved through the right femoral artery for the procedure. During the procedure, TEE in conjunction with angiography was performed for visualization of the exact location of aortic transection (Figure 1). TEE helped in identifying the true lumen from false lumen, and for negotiating the guide wire through the true lumen (Figure 2). TEE also aided in recognizing the lesion that was in the posterior wall of the aorta, which was not identifiable by the standard steep left anterior oblique (LAO) view of aortography. Once the stent was deployed, TEE confirmed its optimal placement sparing the left subclavian artery and ruled out leaks around the stent (Figure 3). Endovascular repair was performed using a self-expanding 28 mm × 100 mm graft stent (Valiant Thoracic Stent Graft with the Captivia Delivery System, Medtronic Vascular, Inc. Santa Rosa, CA). Following the stent deployment and confirmation by TEE, an aortogram was performed that showed proper positioning of the stent with no evidence of endoleak (Figure 4). The following day a spinal CT chest confirmed the stent position with exclusion of aortic false lumen, perfusion of branch vessels and absence of peri-graft leak. The patient was discharged home on the 7th day after the intervention with advice to continue his antihypertensive medication.
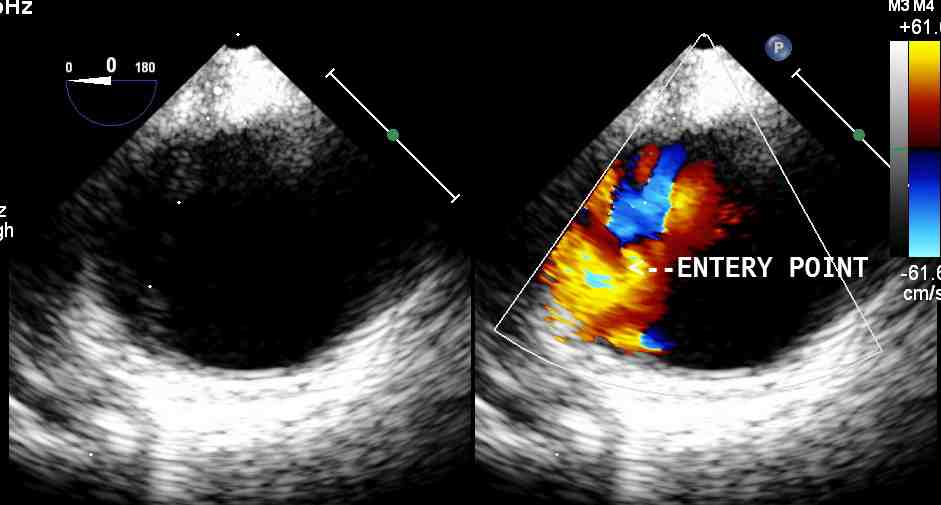
Figure 1: Short axis view of descending aorta showing the location of the aortic transection.
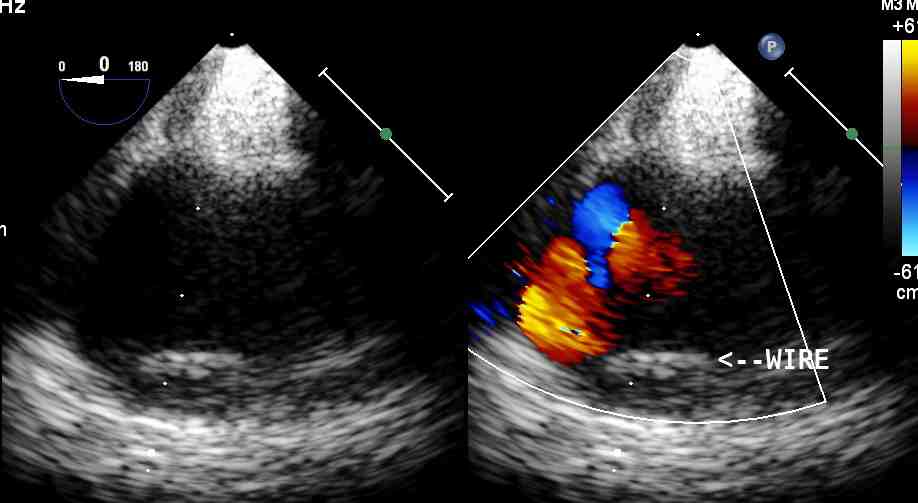
Figure 2: Short axis view of descending aorta showing the location of the guide wire in the true lumen of the aorta.
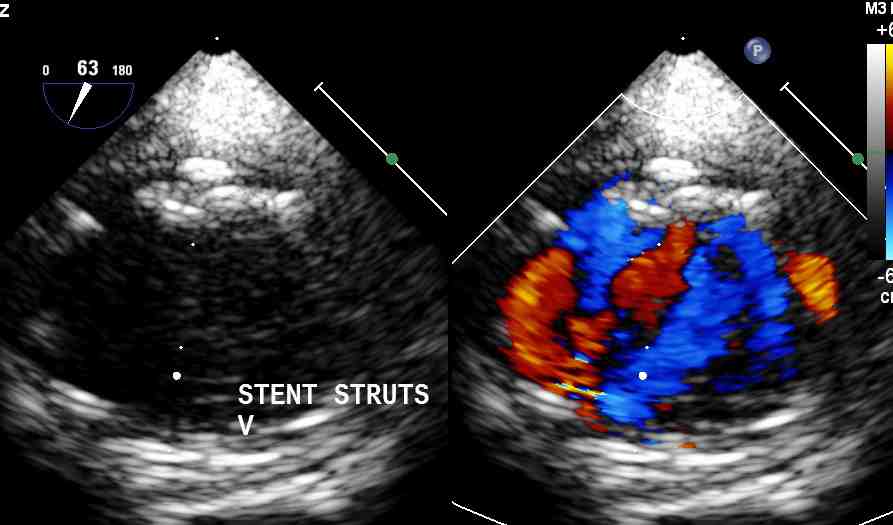
Figure 3: Descending aortic long axis view depicting the upper margin of the stent with no para-stent leaks nor tear.
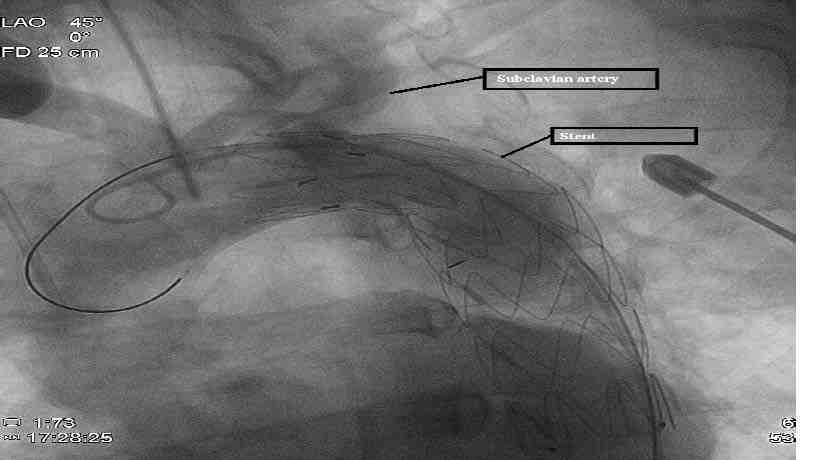
Figure 4: Aortogram of aortic arch depicting optimal position of the stent with no endoleaks and sparing of the left subclavian artery.
Discussion
Chest trauma due to rifle butt recoil resulting in Stanford type A aortic dissection that was managed by surgery has been previously described.3 This case report describes the management of an aortic transection caused by a motor vehicle accident by an endovascular intervention thereby avoiding surgery.
Motor vehicle accidents result in severe decelerating injuries leading to blunt thoracic aortic injuries.4 The aortic segment just distal to the left subclavian artery is the common site of injury.5 Aortic rupture occurs within 24 hours in 33% of the patients who reach the hospital but have no decisive procedure done.4 Therefore, either a surgical or an endovascular intervention has to be done at the earliest in such patients in order to avoid aortic rupture. Open surgical repairs have high mortality and morbidity.4 Currently, the management paradigm for thoracic aortic injury has changed in favor of endovascular repair as it may be performed with lower peri-operative morbidity and mortality when compared with standard open repair.1
TEE has an important role to play in the management of traumatic aortic transections. It has a high degree of sensitivity [~ 100%] and 98% specificity in the detection of aortic injury.6 TEE guidance is considered appropriate in endovascular thoracic aortic procedures for monitoring, procedural guidance, and/or endovascular graft leak detection (Class IIa, Level of Evidence: B).7 It has been suggested that TEE is a valuable tool for the detection of endovascular repair related procedural complications including access complications, endoleaks, coverage of the left subclavian artery and stent collapse.8
In comparison with fluoroscopy and angiography, despite the fact that TEE is used as a supplementary imaging technique to these two, it is also considered superior to both as it can identify proper positioning of the guide wire in the true lumen as against false lumen and aids in the detection of proximal stent leaks by color Doppler studies. Based on the pulse wave Doppler velocity with a cutoff value of 50 cm/sec, TEE may be useful for differentiating true leaks from graft porosity. Endoleaks and new proximal and distal intimal tears in the thoracic aorta after stent –graft implantation are better identified by TEE as compared with angiography.9 TEE can diagnose residual flow into the false lumen reliably as compared to intraoperative angiography and can demonstrate exclusion of subclavian artery.10
TEE helped by exactly localizing the partial transection site with minimal contrast administration in the patient. The origin of the left subclavian artery was spared under TEE guidance that was later confirmed by angiography. Coverage of the left subclavian artery origin by the stent was possible as the lesion was very close. In addition, coverage of the left subclavian without revascularization during endovascular repair of thoracic aortic pathologies was suggested by EUROSTAR investigators as the most significant independent predictor of spinal cord ischemia.11 This problem was also avoided under TEE guidance.
However, there are limitations to the role of TEE in endovascular stent repair of aortic transections. The TEE probe may have to be drawn into upper esophagus to allow fluoroscopy during graft deployment. Echo density of the graft creates echo dropouts and as the stents are echo reflectors, it can interfere with proper imaging. Evaluation of proximal aortic arch is unsatisfactory due to poor acoustic windows. Lesions of the distal ascending aorta are difficult or impossible to visualize with TEE, secondary to interference of the trachea and left main stem bronchus. Examination of the subdiaphragmatic descending aorta is also limited. Ultrasonic artifacts sometimes are confused with intimal tear and lead to an inappropriate diagnosis. Despite these limitations, TEE is a widely used perioperative and intraoperative diagnostic modality for evaluating patients with aortic injuries.8
After three months, the endovascular stent recipient was doing well, and his blood pressure was well controlled. As the patient had a spiral CT prior to discharge, he needs further follow-up at 6 and 12 months intervals. In addition to annual clinical follow-up and as per the guidelines of the European Association of Cardio-Thoracic Surgery,2 he should be followed-up with computed tomography angiography and magnetic resonance imaging.
Conclusion
TEE helped in identifying the exact location of the aortic lesion in the patient and aided in the ideal placement of the endograft sparing the left subclavian artery origin. In conclusion, TEE is a valuable tool in the management of endovascular repair of aortic pathologies as it enhances the information provided by aortic imaging, helps in sizing and accurate placement of the endograft, and identifies graft related problems like endoleaks and stent collapse. TEE also helps in solving diagnostic conundrums during endovascular intervention.
Acknowledgements
The author reported no conflict of interest and no funding was received on this work.
References
1. Clouse WD. Endovascular repair of thoracic aortic injury: current thoughts and technical considerations. Semin Intervent Radiol 2010 Mar;27(1):55-67.
2. Grabenwöger M, Alfonso F, Bachet J, Bonser R, Czerny M, Eggebrecht H, et al; European Association for Cardio-Thoracic Surgery (EACTS); European Society of Cardiology (ESC); European Association of Percutaneous Cardiovascular Interventions (EAPCI). Thoracic Endovascular Aortic Repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg 2012 Jul;42(1):17-24.
3. Rao M, Panduranga P, Al-Mukhaini M, Al-Jufaili M, Valiath J. Ischemic stroke secondary to aortic dissection following rifle butt recoil chest injury: a case report. Oman Med J 2011 Nov;26(6):438-440.
4. Borsa JJ, Hoffer EK, Karmy-Jones R, Fontaine AB, Bloch RD, Yoon JK, et al. Angiographic description of blunt traumatic injuries to the thoracic aorta with specific relevance to endograft repair. J Endovasc Ther 2002 Jun;9(Suppl 2):II84-II91.
5. Kato N, Dake MD, Miller DC, Semba CP, Mitchell RS, Razavi MK, et al. Traumatic thoracic aortic aneurysm: treatment with endovascular stent-grafts. Radiology 1997 Dec;205(3):657-662.
6. Smith MD, Cassidy JM, Souther S, Morris EJ, Sapin PM, Johnson SB, et al. Transesophageal echocardiography in the diagnosis of traumatic rupture of the aorta. N Engl J Med 1995 Feb;332(6):356-362.
7. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American College of Radiology; American Stroke Association; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of Thoracic Surgeons; Society for Vascular Medicine. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010 Apr;121(13):e266-e369.
8. Metaxa V, Tsagourias M, Matamis D. The role of echocardiography in the early diagnosis of the complications of endovascular repair of blunt aortic injury. J Crit Care 2011; 26:434.e7-12.
9. Rocchi G, Lofiego C, Biagini E, Piva T, Bracchetti G, Lovato L, et al. Transesophageal echocardiography-guided algorithm for stent-graft implantation in aortic dissection. J Vasc Surg 2004 Nov;40(5):880-885.
10. Gonzalez-Fajardo JA, Gutierrez V, San Roman JA, Serrador A, Arreba E, Del Rio L, et al. Utility of intraoperative transesophageal echocardiography during endovascular stent-graft repair of acute thoracic aortic dissection. Ann Vasc Surg 2002 May;16(3):297-303.
11. Buth J, Harris PL, Hobo R, van Eps R, Cuypers P, Duijm L, et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: Incidence and risk factors. a study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) registry. J Vasc Surg 2007 Dec;46(6):1103-1110, discussion 1110-1111.
|