|
Abstract
Objective: This study aimed at evaluating the prevalence, pattern and predisposing factors for hepatic adverse effects with statins in a regional hospital in Sultanate of Oman.
Methods: A retrospective review of the patient files in Department of Medicine during the year 2011 was done to evaluate any hepatic dysfunction possibly related to statins among the patients. For each case of suspected statin induced hepatic effect, additional details on temporal relationship, pattern of presentation, management, final outcome and any contributing factors were obtained. Difference in the occurrence of hepatic effects based on the patient demographics and drug characteristics was additionally evaluated.
Results: A total of 927 patients meeting the inclusion criteria were included for the study. Mean age of the evaluated patients was 63.1 ± 11.37 and median duration of use of statin in months was 22 (IQR, 43.25). In 40 (4%) of the 927 patients, there was presence of a hepatic effect considered to be statin related and only in 12 (1%) patients a significant transaminase rise (>3 times) was observed. Median duration of use of statin among those patients who developed suspected statin induced hepatic effects and those who did not was 45 (IQR,52) and 21 (IQR, 43) months, respectively and the difference observed was statistically significant. A significant difference in the prevalence of hepatic effects was observed only based on the duration of statin use.
Conclusion: There was an infrequent occurrence of significant hepatic effects associated with statins in the study population. Our results support the latest recommendations including from United States Federal Drug Administration (US FDA) that statins appear to be associated with a very low risk of serious liver injury and that routine periodic monitoring of transaminases does not appear to detect or prevent serious liver injury in association with statins.
Keywords: statins, hepatic effects, Sultanate of Oman, retrospective review.
Introduction
Coronary heart disease (CHD) is the leading cause of morbidity and mortality worldwide and lowering of cholesterol levels has been shown to reduce cardiovascular events.1,2 Statins are the drugs of choice for patients with hypercholesterolemia and other risk factors for CHD.3,4 Several clinical studies and meta-analyses have shown the beneficial effects of lipid lowering treatment using statins in primary and secondary prevention of cardiovascular disease.5-7 Statins are currently one among the most frequently used and largest selling prescription drug worldwide including Sultanate of Oman.8-10
Since their introduction in 1980s there were concerns regarding the uncommon but significant side effects of statins on the liver.11 Asymptomatic mild elevation of serum transaminases (often self-limiting) have been reported with all statins with varying incidence.12-14 Further, it is reported that only 3% of patients with early, minor elevation in serum transaminases experience a subsequent persistent significant elevation (greater than three times the upper limit of normal).15 This elevation is most often transient and will resolve spontaneously in 70% of cases even if the statin and dose are continued unchanged.16-18 It is worth to note that, in addition to the harmless elevations of transaminases with statins, there have always been reports to suggest more serious hepatotoxicity extending to fatal reactions.14,19-22 This potential for hepatotoxic effects has received due attention along with the other significant effect of statins; myopathy.
Routine monitoring of liver function tests (LFTs) were included in the recommendations from manufacturers and other sources while patients are initiated and continued on statins. For instance, manufactures recommended systematic and frequent monitoring of LFTs when treatment is first started or the dose is increased, then repeated at 12 weeks and monitored every 6 months.23 During the recent years, there have been conflicting data on the usefulness of this routine monitoring of LFTs while patient is on statin.24,25 In 2012, United States Food and Drug Administration (US FDA) after reviewing the current monitoring guidelines, National Lipid Association’s (NLA’s) recommendations,24,26 and other post marketing data, determined that routine periodic monitoring of liver enzymes does not appear to detect or prevent the liver injury caused by statins. Hence, healthcare professionals should perform liver enzyme tests before initiating statin therapy and as clinically indicated thereafter.27
Statins are one of the commonly used drugs among patients in Oman.10 Studies in the field of drug safety are limited in Sultanate of Oman as well as in Middle East. There is only limited data on the safety profile of statins based on studies conducted in Oman or Middle East.28 Along with the data reported elsewhere, it is important to study in the local populations due to variations in frequency and pattern of adverse drug reactions (ADRs) in different populations. Hence, this study aimed at estimating the occurrence and pattern of hepatic adverse effects with statins with an evaluation of the predisposing factors. It was considered important to observe the outcome of any observed hepatic effects in the affected patients. We considered that the obtained information would provide opportunities for interventions to promote safer drug use and be a useful addition to the existing literature on these aspects.
Methods
The study received approval from the Regional Research and Ethics Committee of Al Dakhliya governorate, Ministry of Health. A retrospective review of the medical records was done to identify patients on any statin agent during the year 2011 (the study sample) in Nizwa Hospital; a 302 bed regional hospital. Inclusion criteria for the study included data of patients who visited the outpatient department or were admitted as inpatients in Department of Medicine and was receiving any HMG Co-A reductase inhibitors (statins) in their medication list. Moreover, only the patients, who had received statin drug for more than a month and have majority of follow ups in Nizwa Hospital or the Polyclinic associated with it, were selected for the study. Patient files with missing data or non-evaluable details were excluded. For the selected cases, details on patient demographics, disease and drug details were collected. Indication for use of statin was identified based on patient's file and details of laboratory data for monitoring liver function were documented.
Each patient’s file was critically reviewed for presence of any documentation of hepatic dysfunction or abnormal LFTs. For the purpose of the study, any level of elevation of transaminases or bilirubin above the normal values was considered as hepatic abnormality and was further reviewed. Any symptomatic or laboratory hepatic dysfunction was thoroughly evaluated to identify any possible contribution from statin in the hepatic effect. Further specifically, each of the patient file was reviewed for presence of any documentation on suspected statin induced hepatic adverse effects. Suspected statin induced hepatitis was considered if the hepatic effect developed during the use of statin and there was a suspicion that use of the drug has a contribution to the observed effect; either as the sole or one among the contributing factors. Temporal relationship, dechallenge and rechallenge information where ever available contributed to the causality assessment of statin induced hepatic effect. In those cases where there was a strong suspicion that another aspect (example hepatitis infection, CHF, etc) was the most likely factor; it was not considered as a suspected statin induced effect. In those with any suspected statin induced hepatic effects, additional details on the temporal relationship, presentation, management of hepatic effects, final outcome and any contributing factors were evaluated. Difference in the prevalence of hepatic effects based on the patient demographics as well as based on the drug characteristics was additionally evaluated. Additionally, the frequency of liver function monitoring in the patients was assessed. The results were statistically analyzed using Statistical Package for Social Sciences (SPSS); version 15. Comparison between groups was done by Chi-Square analysis. Difference was considered statistically significant for p value <0.050.
Results
Among the total of 7497 patients who were admitted or had visited the Department of General Medicine during the year 2011; 1361 patients (18%) received an HMG Co-A reductase inhbitor. From the 1361 patients, 927 were included for the evaluation purpose in consideration with the inclusion and exclusion criteria. A significant percentage of them were in the age group of 60-75 (45%) years and the majority was males (56%) as shown in Table 1. The mean age of the patients was 63.1 ± 11.37. Very few patients had the reported risk factors for development of statin induced hepatitis such as history of alcohol intake (1%), history of liver disease (1%) and history of persistent elevation of transaminases (3%). Median duration of use of statin in months was 22 (Inter Quartile Range (IQR), 43.25. In 94% of the patients, there was no increase in the dose of statin at any time during the follow up.
In vast majority of patients (98%), simvastatin was the statin agent used and 20 mg was the initiating dose (97%); shown in Table 2 and Table 1. Secondary prevention of cardiovascular disease (CVD) was the most common indication for the use of statin.
Evaluating the observation of LFTs, which was in a vast majority of them (85%), was done before initiation of statins adhering to the recommended guidelines (Table 3). In 18% of the patients, LFT monitoring was not performed during the follow-up period. Among the 56 (68%) patients in whom there was an increase in dose, LFT monitoring was done before increasing the dose.
Table 1: Prevalence of suspected statin induced hepatitis in various patient groups.
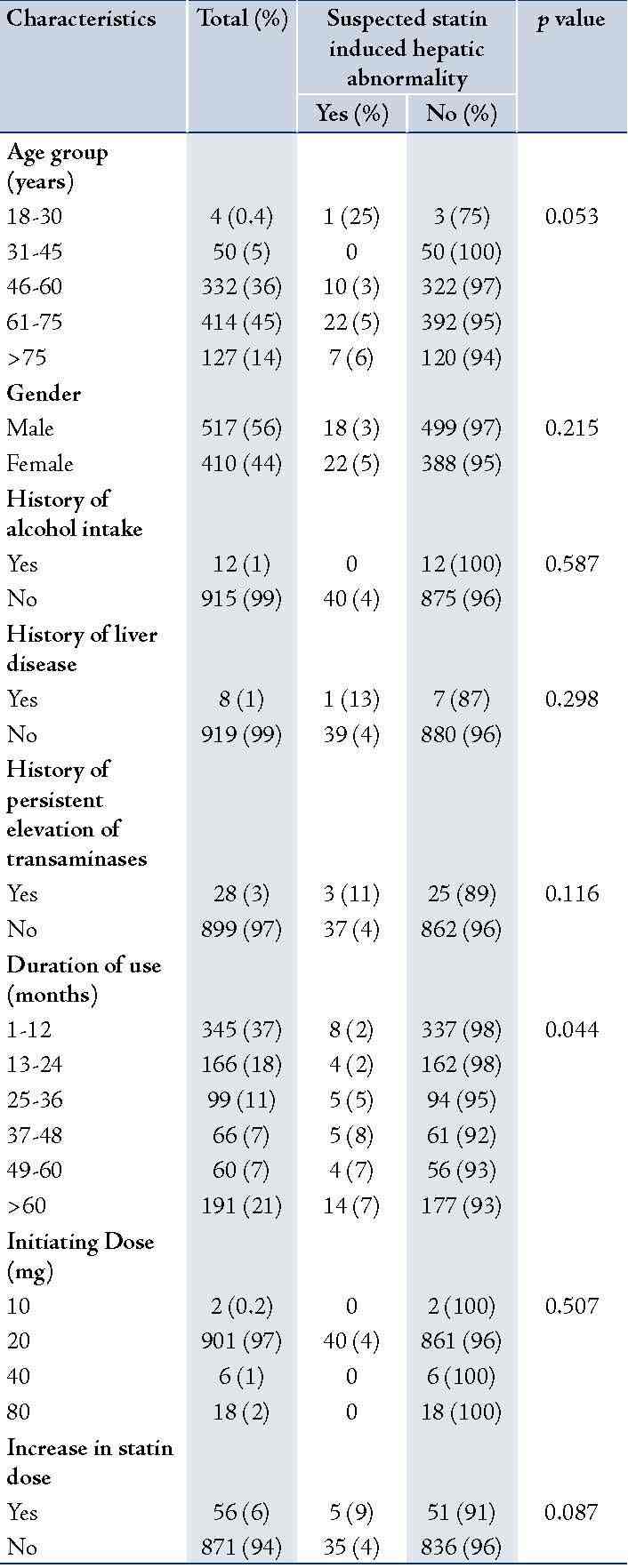
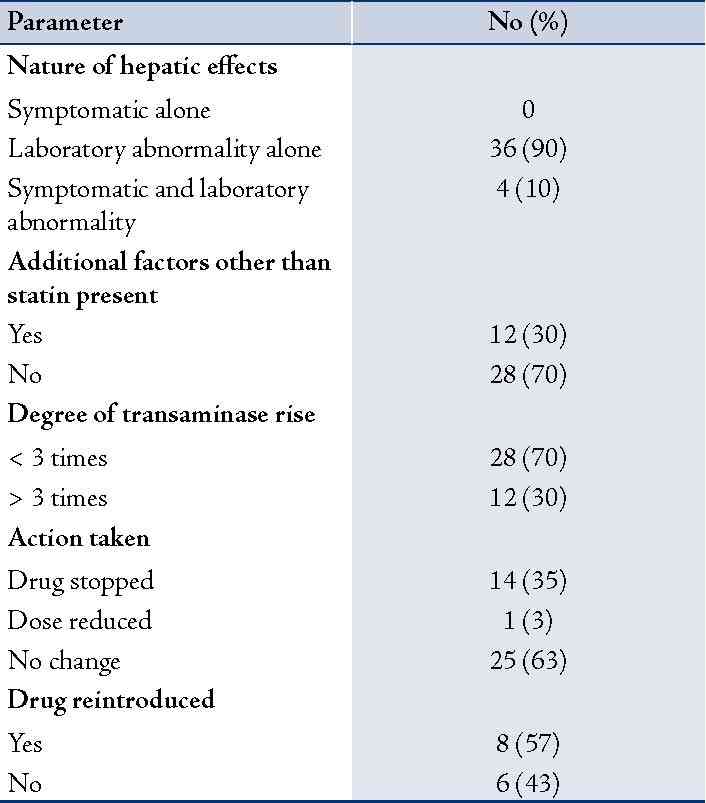
In 132 (14%) of the 927 patients, there was a hepatic abnormality of some degree during the period of time using statin. However, only in 40 of these patients the hepatic effect was considered to be statin related, and in only 12 there was a significant transaminase rise (>3 times the upper limit of normal). Accordingly, the prevalence of suspected statin induced hepatic effect was 4% and that of significant transaminase rise only in 1% of the study population. Evaluating the details of the suspected adverse reaction, majority of them developed a laboratory abnormality alone (90%); Table 4. Suspected adverse reaction resulted in a change in statin regimen (withdrawal of drug or dose reduction) only in 2% of the total patient population.
Table 2: Details of statin drug used and indication for use.
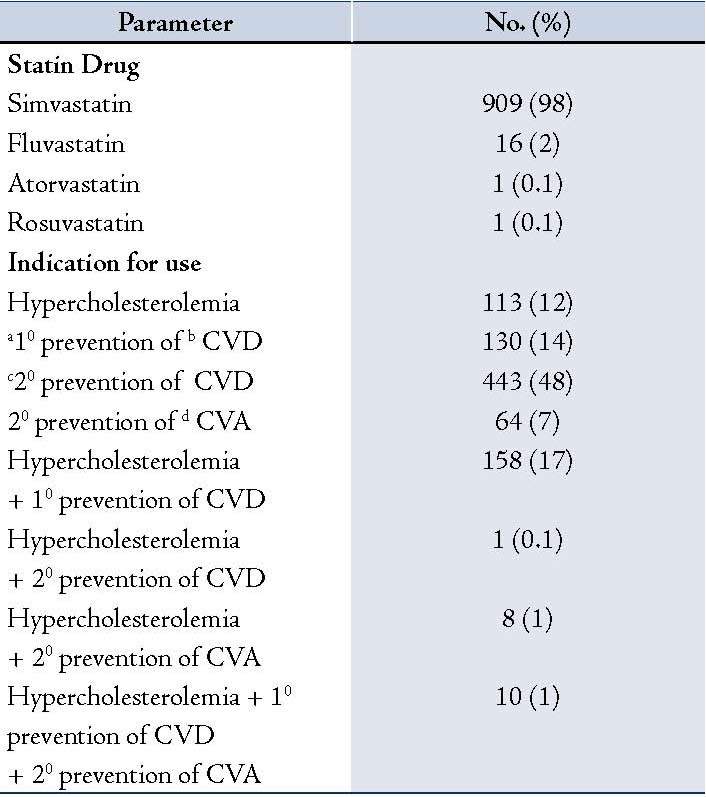
a10 - Primary; b CVD- Cardiovascular disease; c 20 - Secondary; d CVA- Cerebrovascular accident
Table 3: Monitoring of Liver functions and related observations.

Table 4: Nature of suspected statin induced hepatic effects and action taken.
Discussion
Statin use and the associated decrease in the Low Density Lipoprotein (LDL) cholesterol levels are of extreme importance for patient groups because of the additional benefits of cholesterol lowering on primary and secondary prevention of CHD.2,6 Adverse effects on the liver is one of the most commonly known side-effects reported with statins.29 Types of liver injury associated with statin use includes, asymptomatic elevations in aminotransferases (0.1-3%), clinically significant acute liver injury (very rare), fulminat hepatic failure (extremely rare with isolated case reports) and autoimmune hepatitis (case reports).30 The most common presentation is asymptomatic elevations in aminotransferases, a phenomenon called as transaminitis.31,32 Even though risk of serious or persistent hepatic damage with statins in clinical practice is low, idiosyncratic liver injury associated with statins could be severe and hence has to be given due importance.4,26
Since hepatotoxicity was one of the common side effects that was supposed to be monitored in patients according to the product literature as well as other recommendations, unfounded safety concerns about hepatotoxicity are commonly identified among physicians even though not all health care professionals are overly concerned about the hepatic effects of statins.29 Many physicians are reluctant to start statins in patients with an out-of-range liver enzymes value and this reluctance to initiate or interruption of the therapy with statins, leads to dyslipidemia and its grave consequences.29
Based on the present study results, it was observed that statins are one of the most commonly used drugs among the study population, as nearly 20% of patients in internal medicine were on a statin agent. Simvastatin was the most commonly used statin agent since it was the one available for unrestricted use from the Ministry of Health, Sultanate of Oman during the study period. In the present study, interestingly, in 94% of the patients there was no increase in the dose of statin at any time during the follow up.
Among the 132 patients who developed a hepatic abnormality of any degree during the period of statin use, only in 40 (4%; 40/927) of them was the hepatic effect considered to be statin related. In those patients where the LFT abnormality was not considered to be statin related, the most common other cause was congestive heart failure. Unless this important step of excluding other possible causes of hepatic abnormality is done, this may result in unnecessary discontinuation of statin or reduction in dose.30,33
Accordingly, the prevalence of suspected statin induced hepatic effect was 4% in the study population and significant transaminase rise (> 3 times) was observed only in 1% of the patients. There is varying data on the incidence of liver abnormalities with statins. It is reported that elevated aminotransferase levels across multiple studies with different kind of statins did not exceed 3% of the studied patients.24,26,34-36 Incidence of true liver injury caused by statin is noticed to be about 1%.37
Among the evaluated patients, suspected statin induced hepatitis resulted in a change in statin regimen (withdrawal of drug or dose reduction) only in 2% of the patients. Earlier studies have reported that persistent elevation in serum transaminases to more than 3 times the upper limit of normal have occurred in about 1 to 2% of simvastatin patients and requiring withdrawal or interruption of therapy in approximately 1%.16 It has been suggested that it would be prudent to think twice before interrupting statin therapy out of fear of hepatotoxicity.38 Among those cases in which the transaminase elevation persists, the physician must cautiously decide the next action; whether to reduce the dose of the statin, switch to a different statin, or discontinue statin therapy entirely based on an individual assessment of risks and benefits.31,39
Several factors may place patients at increased risk for hepatotoxicity with statins; starting with high or increasing doses of statins, preexisting hepatitis, advanced age and chronic illness.40,41 It was reported that serious hepatic reactions with atorvastatin tend to occur more commonly in females.14 Concurrent use of hepatotoxic substances including acetaminophen, alcohol, fibrates, niacin, macrolide antibiotics, azole antifungals, cyclosporine, and calcium channel blockers may put the patient at a higher risk of hepatotoxicity with statins.39 The interactions between alcohol intake and statin treatment have been poorly studied.31 On the other hand, there are reports that the effect of ageing on the risk of hepatic damage with statins is not clearly known.42 In the present study, although the prevalence of suspected hepatic effects with statins were higher in females, those in the advanced age group, those with a history of persistent elevation in liver enzymes and those in whom there was increase in statin dose; the differences observed were not significant. A significant difference in the prevalence of hepatic effects was observed only based on the duration of statin use.
Considering the prognosis of statin induced hepatic effects, according to some post marketing surveillance studies, approximately 70% of these statin induced elevations in transaminases will spontaneously fall back into the normal range even as treatment continues.39 In other cases, these elevations are usually reversible with a dose reduction.43
Based on the collective evidence available from various sources, it is clear that only very rarely elevations in transaminase levels seen in low to moderate dosages of statins progress to liver failure.13,44,45 Most recent literature in this aspect by Younoszai et al where they used the US third national health and nutrition examination survey (NHANES III)- mortality linked files to assess the association between statin use and liver related mortality also supports the same. It was reported that after a decade of follow up, there was no association between statin use and liver related mortality. In fact, the rate of liver related mortality was significantly lower among statin users compared to non-statin users.46 US FDA conducted a review of its post-marketing data to evaluate the risk of clinically serious hepatotoxicity associated with statins by searching the Agency’s Adverse Event Reporting System (AERS) database. Accordingly, it was published that reporting of statin-associated serious liver injury to the AERS database was extremely low (reporting rate of ≤2 per one million patient-years). FDAs conclusion was that even though there has been a rising use of statins as a class since the late 1990s, there has not been a detectable increase in the annual rates of fatal or severe liver injury cases possibly or probably causally associated with statin use.27
The need of getting a baseline LFT before starting statin has been recommended from time of introduction of these agents in the market and recommendations still exists.27 Following the recommendations, LFTs were done before initiation of statins in vast majority (85%) of evaluated patients. It was observed that in 18% of the patients, there was no monitoring of LFT after initiation of statin. In the study conducted by Smith et al, in 15% of patients on statins, transaminase levels were not checked during the one year of follow up.25
Usefulness of the routine monitoring of LFTs after initiation of statins was a matter of discussion in recent years and many were advocating that there is no need for such routine monitoring.24,26 Considering the low incidence of statin induced true liver injury, it was reported based on evidence that one would have to monitor transaminase levels in 100,000 patients each year for an average of 3 years to detect 110 patients who have consecutive elevations in ALT in order to identify the statistical 0.1 person who may experience liver failure.18 Smith et al reported that if the actual risk is sufficiently low, the cost of screening, evaluation of minor test result abnormalities and drug discontinuation could be minimised.25 The low prevalence of clinical significant statin induced hepatic effects observed in our study supports the label changes for statins approved by US FDA that ‘healthcare professionals should perform liver enzyme tests only before initiating statin therapy and as clinically indicated thereafter. FDA recommends that 'if serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment, therapy should be interrupted. If an alternate aetiology is not found, the statin should not be restarted’.27
Another clinical dilemma is use of statins in patients with existing baseline elevations of serum liver enzyme levels or liver disease. Presence of baseline elevations of serum liver enzyme levels is frequently secondary to associated co-morbid conditions; dyslipidemia, obesity, and diabetes mellitus which shares features of non-alcoholic fatty liver disease (NAFLD). Studies support that statins can generally be used safely in patients with NAFLD with appropriate monitoring.31 It is reported that statins appear to be safe in patients with chronic hepatitis B and C as is the case with stable non cirrhotic or compensated cirrhosis from other causes. But statin pharmacokinetics and metabolism may be altered with abnormally high serum levels in those patients with more extensive hepatic impairment.31 It is interesting to note that some recent studies demonstrated that in fact, statin treatment may improve liver enzyme levels as well as hepatic steatosis.47,48 On the same note, it is clinically wise to consider that statins as mentioned previously elsewhere in the manuscript do possess the risk of developing significant hepatic effects. Hence, this adverse effect have to be given importance before initiating statin, while educating the patient, during patient follow ups and of course as a differential diagnosis if a patient on statin develops hepatic effects.
Major limitation of the present study is based on retrospective data and hence, like any other study relying on already available data, inherent drawbacks of incomplete or inconsistent documentation could be expected. Prevalence of liver function abnormalities was estimated based on the data in the patient records, and in many of the patients there were no liver function tests done during routine follow ups. Considering the awareness of clinicians on the reported risks of hepatitis, it is likely that any patient with any significant suspicion of hepatic effects might have been tested and identified in due course. The impact of higher doses of statin could not be assessed as only in minority of patients there was increase in dose at any time during treatment. As vast majority of the patients were only on simvastatin, a reliable data on the effect of other agents could not be assessed. As the relation between statin and the liver function abnormality observed in the patient was evaluated based on assessment at a later stage, inherent draw backs of causality assessment of retrospective data should be borne in mind. Even with these limitations, we consider that the study was useful in obtaining valuable data on the prevalence of statin induced hepatic effects among the local population. Furthermore, this study will be a useful initiative in conducting larger studies in the field of drug safety among Omani population.
Conclusion
The low prevalence and pattern of presentation of hepatic effects with statins in the study population was more or less similar to what has been already reported in literature. Even though asymptomatic elevations in transaminases were seen more frequently, symptomatic manifestations were infrequent in the study population. Duration of use of statin was the only factor which was significantly associated with the development of hepatic effects among the patients. The infrequent development of statin induced significant hepatic effects observed in our study support the latest data including that from US FDA ‘all currently marketed statins appear to be associated with a very low risk of serious liver injury and that routine periodic monitoring of serum alanine aminotransferase does not appear to detect or prevent serious liver injury in association with statins’. Changes in product label for statins approved by US FDA ‘to remove the need for routine periodic monitoring of liver enzymes in patients taking statins, to perform liver enzyme tests before starting statin therapy and as clinically indicated’ is a typical example of the benefit of post marketing surveillance in making need based recommendations for the way drugs are used in routine practice. Concurrently in daily practice, we should not overlook the potential of statins to cause significant and serious hepatic effects as reported in literature. More of similar studies on safety of drugs, especially the common ones should be conducted in this country which will supplement the existing data of individual drugs worldwide and identify any difference in the prevalence and pattern of occurrence of these effects in the local population. Such studies will definitely improve the pharmacovigilance activities in the country to contribute to the level it is expected to be.
Acknowledgments
The project was funded by The Research Council (TRC), Oman under the grant number ORG/HSS/11/008 as part of the Open Research Grant (ORG) scheme. We would like to greatly acknowledge TRC, Oman for the support granted. Further, we would like to express our gratitude to the Hospital Administration, Nizwa Hospital for granting permission to conduct the study as well as the Computer department for providing the necessary details for planning and conduct of the study.
References
1. Centers for Disease Control and Prevention (CDC). Leading causes of death. FastStats Website. http://www.cdc.gov/nchs/FASTATS/lcod.htm. Accessed on November 12, 2013.
2. Grundy SM, Cleeman JI, Merz CNB. For the Coordinating committee of the National Cholesterol Education Program. Implications of recent trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110-227-39.
3. Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, et al; Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001 Apr;285(13):1711-1718.
4. Björnsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol 2012 Feb;56(2):374-380.
5. Wei L, Ebrahim S, Bartlett C, Davey PD, Sullivan FM, MacDonald TM. Statin use in the secondary prevention of coronary heart disease in primary care: cohort study and comparison of inclusion and outcome with patients in randomized trials. BMJ2005;330:821.
6. Executive Summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA2001;285:2486-97.
7. Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol 2008 Nov;52(22):1769-1781.
8. Mills EJ, O’Regan C, Eyawo O, Wu P, Mills F, Berwanger O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40 000 patients. Eur Heart J 2011 Jun;32(11):1409-1415.
9. Mitka M. Expanding statin use to help more at-risk patients is causing financial heartburn. JAMA 2003 Nov;290(17):2243-2245.
10. Al Shukry A, Rashed W, Zubaid M. The use of evidence-based therapy in acute myocardial infarction patients admitted to hospital during the Gulf Registry of Acute Coronary Events (Gulf RACE). Heart Views 2009;10(1). http://www.hmc.org.qa/heartviews/vol10No1/02ORIGINAL_ARTICLE1.html.
11. Armitage J, Bowman L, Collins R, Parish S, Tobert J; MRC/BHF Heart Protection Study Collaborative Group. Effects of simvastatin 40 mg daily on muscle and liver adverse effects in a 5-year randomized placebo-controlled trial in 20,536 high-risk people. BMC Clin Pharmacol 2009;9:6.
12. Bolego C, Baetta R, Bellosta S, Corsini A, Paoletti R. Safety considerations for statins. Curr Opin Lipidol 2002 Dec;13(6):637-644.
13. Bernini F, Poli A, Paoletti R. Safety of HMG-CoA reductase inhibitors: focus on atorvastatin. Cardiovasc Drugs Ther 2001;15(3):211-218.
14. Clarke AT, Mills PR. Atorvastatin associated liver disease. Dig Liver Dis 2006 Oct;38(10):772-777.
15. Black DM, Bakker-Arkema RG, Nawrocki JW. An overview of the clinical safety profile of atorvastatin (lipitor), a new HMG-CoA reductase inhibitor. Arch Intern Med 1998 Mar;158(6):577-584.
16. Boccuzzi SJ, Bocanegra TS, Walker JF, Shapiro DR, Keegan ME. Long-term safety and efficacy profile of simvastatin. Am J Cardiol 1991 Nov;68(11):1127-1131.
17. Bays H. Statin safety: an overview and assessment of the data–2005. Am J Cardiol 2006 Apr;97(8A):6C-26C.
18. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol 2006 Apr;97(8A):52C-60C.
19. Nakad A, Bataille L, Hamoir V, Sempoux C, Horsmans Y. Atorvastatin-induced acute hepatitis with absence of cross-toxicity with simvastatin. Lancet 1999 May;353(9166):1763-1764.
20. Perger L, Kohler M, Fattinger K, Flury R, Meier PJ, Pauli-Magnus C. Fatal liver failure with atorvastatin. J Hepatol 2003 Dec;39(6):1095-1097.
21. Ridruejo E, Mandó OG. Acute cholestatic hepatitis after reinitiating treatment with atorvastatin. J Hepatol 2002 Jul;37(1):165-166.
22. Geoghegan M, Smith V, Green JR. Acute cholestatic hepatitis associated with atorvastatin. Gut 2004;53(Suppl. III):A123.
23. Parke-Davis. Lipitor (atorvastatin calcium) tablets prescribing information. New York, NY; 2007 Nov.
24. McKenney JM, Davidson MH, Jacobson TA, Guyton JR; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol 2006 Apr;97(8A):89C-94C.
25. Smith CC, Bernstein LI, Davis RB, Rind DM, Shmerling RH. Screening for statin-related toxicity: the yield of transaminase and creatine kinase measurements in a primary care setting. Arch Intern Med 2003 Mar;163(6):688-692.
26. Cohen DE, Anania FA, Chalasani N; National Lipid Association Statin Safety Task Force Liver Expert Panel. An assessment of statin safety by hepatologists. Am J Cardiol 2006 Apr;97(8A):77C-81C.
27. Food and Drug Administration. FDA drug safety communication: Important safety label changes to cholesterol-lowering statin drugs. Rockville, MD; 2012 Feb 28. Available at URL http://www.fda.gov/drugs/drugsafety/ucm293101.htm. Accessed on 30 th April 2012.
28. Al-Siyabi K, Farhan H, Al-Rasadi K, Al-Salhi A, Al-Hinai AT, Al-Zakwani I. Safety of simvastatin and goal attainment for low-density lipoprotein cholesterol in sultan qaboos university hospital. Oman Med J 2010 Oct;25(4):264-268.
29. Kon RH, Russo MW, Ory B, Mendys P, Simpson RJ Jr. Misperception among physicians and patients regarding the risks and benefits of statin treatment: the potential role of direct-to-consumer advertising. J Clin Lipidol 2008 Feb;2(1):51-57.
30. Bhardwaj SS, Chalasani N. Lipid-lowering agents that cause drug-induced hepatotoxicity. Clin Liver Dis 2007 Aug;11(3):597-613, vii.
31. Calderon RM, Cubeddu LX, Goldberg RB, Schiff ER. Statins in the treatment of dyslipidemia in the presence of elevated liver aminotransferase levels: a therapeutic dilemma. Mayo Clin Proc 2010 Apr;85(4):349-356.
32. Dujovne CA. Side effects of statins: hepatitis versus "transaminitis"-myositis versus "CPKitis". Am J Cardiol 2002 Jun;89(12):1411-1413.
33. Mancini GB, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: proceedings of a Canadian Working Group Consensus Conference. Can J Cardiol 2011 Sep-Oct;27(5):635-662.
34. Pasternak RC, Smith SC Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. Circulation 2002 Aug;106(8):1024-1028.
35. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008 Nov;359(21):2195-2207.
36. Stein EA, Amerena J, Ballantyne CM, Brice E, Farnier M, Guthrie RM, et al. Long-term efficacy and safety of rosuvastatin 40 mg in patients with severe hypercholesterolemia. Am J Cardiol 2007 Nov;100(9):1387-1396.
37. Gillett RC Jr, Norrell A. Considerations for safe use of statins: liver enzyme abnormalities and muscle toxicity. Am Fam Physician 2011 Mar;83(6):711-716.
38. Sikka P, Saxena KK, Kapoor S. Statin hepatotoxicity: Is it a real concern? Heart views 2011’ 12 (3): 104-6.
39. Tolman KG. The liver and lovastatin. Am J Cardiol 2002 Jun;89(12):1374-1380.
40. Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation. 2004; 109(23 Suppl 1):III 50-57.
41. Stollab D, Dariolia R, Rodondia N. Lipid-lowering therapies and liver enzymes Kardiovaskuläre Medizin 2009;12(9):239–244.
42. Hilmer S, Gnjidic D. Statins in older adults. Australian Prescriber 2013;36(3):79-82.
43. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the national cholesterol education program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002 Dec;106(25):3143-3421.
44. de Denus S, Spinler SA, Miller K, Peterson AM. Statins and liver toxicity: a meta-analysis. Pharmacotherapy 2004 May;24(5):584-591.
45. Wlodarczyk J, Sullivan D, Smith M. Comparison of benefits and risks of rosuvastatin versus atorvastatin from a meta-analysis of head-to-head randomized controlled trials. Am J Cardiol 2008 Dec;102(12):1654-1662.
46. Younoszai Z, Li Z, Stepanova M, Erario M, Cable R, Younossi ZM. Statin use is not associated with liver related mortality. Ann Hepatol 2013 Jan;13(1):84-90.
47. Hyogo H, Tazuma S, Arihiro K, Iwamoto K, Nabeshima Y, Inoue M, et al. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism 2008 Dec;57(12):1711-1718.
48. Argo CK, Loria P, Caldwell SH, Lonardo A. Statins in liver disease: a molehill, an iceberg, or neither? Hepatology 2008 Aug;48(2):662-669.
|