| |
Abstract
Objectives: Stillbirth is an important public health concern and its rate indicates the sanitary development of society. The purpose of this study is to determine the trend of stillbirth rates and its risk factors in Babol.
Methods: A retrospective study was conducted based on the data of hospital charts of two major Gynecological wards in Shahid Yahyanejat and Babol clinic hospitals in Babol, Northern Iran. In the first phase, the frequencies of stillbirths and live birth deliveries were collected for the period of 1999-2008. In the second phase, a case-control study of 150 stillbirths cases and 300 live births as controls was conducted. The risk factors data included maternal age, gestational age, gravity, history of stillbirth, abortion, diabetes mellitus, preeclampsia, fetal sex, residence area, birth interval and prenatal care. The odds ratio for risk factors with 95% confidence interval for stillbirths was calculated using the logistic regression model.
Results: Stillbirth rate was reduced significantly from 10.51 in 1999 to 8.57 per 1000 deliveries in 2008 (p=0.001). A significant association was found between preterm delivery (p=0.001) and preeclampsia (p=0.01) with stillbirths. Although the proportion of stillbirths was higher among mothers with history of diabetes, abortion and maternal age of more than 35 years, the odds ratio was not statistically significant.
Conclusion: There is a relationship between stillbirth, preterm delivery and preeclampsia. Thus, we can considerably prevent stillbirths with sanitary remedial interference on these risk factors.
Keywords: Trend; Stillbirth; Preterm delivery; Preeclampsia; Diabetes; Maternal age.
Introduction
Fetal death relates to the intrauterine death of a fetus at any gestational age. It occurs prior to the complete expulsion or extraction from its mother irrespective of duration of pregnancy, where the fetus does not breath or show any other evidence of life such as heart pulse rate, pulsation rate of umbilical cord or definite movement of voluntary muscles, and its rate is an indicator of sanitary and social development.1 In low-income and middle-income countries, 50% of stillbirths or more are probably caused by infections.2 World Health Organization (WHO) defined stillbirth as fetal death that occurred in the late period of pregnancy that is defined with gestational age. The definition may vary from 16 weeks to 28 weeks of gestational age in different countries.3,4 The fetal death lower than the threshold is called abortion. A common definition of stillbirth as used by WHO and the National Center for Health Statistics (NCHS) is the fetal death at least 20 weeks of gestation.1,5 The Center for Disease Control and Prevention (CDC) further classified stillbirth as either early or late stillbirth. An early stillbirth is a fetal death occurring at 20-27 weeks and late pregnancy stillbirth occurs between 28 and 36 completed weeks of gestational age.6 Although this categorization is roughly arbitrary, the advantage is to distinguish the early stillbirth that is less preventable from the more preventable late pregnancy stillbirth.
In epidemiologic studies, it has been documented that 3 million stillbirths occur annually worldwide.7,8 Overall, the stillbirth rate is slightly higher than neonatal mortality rate but lower than infant mortality rate.9 However, fetal death with unspecified cause is more common than infant mortality with unspecified cause. In the United States, the rate of stillbirths was reported as 6.41 per 1000 deliveries (stillbirths and livebirths) in 2004.10 While, during two decades in the Islamic Republic of Iran, the rate of stillbirths varied from 12.8 per 1000 deliveries to 40.0 per 1000 deliveries in different regions.11,12
In the Middle East and our neighboring countries, the rate of stillbirths varies from 8 in Qatar to 38 per 1000 births in Afghanistan.13,14 It was reported that the stillbirth, neonatal, early neonatal and prenatal mortality rates in Qatar were very close to the rates found in developing counties and were lower than those reported in some Gulf countries such as Bahrain and Saudi Arabia.13 Another report from WHO collaborating centers in Argentina, Egypt, India, Peru, South Africa and Vietnam, the stillbirth rate was 12.5 per 1000 births and early neonatal mortality rate was 9.0 per 1000 livebirths.15
Several factors have been reported to elevate the risk of stillbirths such as advanced maternal age, multi parity, preeclampsia, gestational diabetes, history of stillbirth and abortion, placental abruption and premature rupture of membrane (PROM) in women aged above 35 years.16,17 Stillbirths related to congenital disorders was almost correlated with maternal age of >35 years. In developing countries, prolonged and obstructed labor, pre-eclampsia and various infections appeared to account for the majority of stillbirths. However, the findings of associated risk factors are controversial.18 They may vary with respect to social, cultural and racial status. In the United States, the risk of stillbirths in black women is two times greater than in white women even with sufficient prenatal care.19,20 The elevated risk in the black race may be attributed to higher rates of diabetes and preeclampsia, PROM and decolmentation compared to white women. In addition, obesity, smoking, infections and blood concentration, as well as medical disorders of mothers were reported to be independent risk factors for stillbirths.21-23 Obese women may have higher rates of stillbirth due to higher rates of diabetes and preeclampsia.
The rural and urban populations of Babol in the southern region of the Caspian sea, northern of Iran, were covered by a health network system and the majority of deliveries were carried out in two hospitals under study and the rate of home deliveries was close to zero in the studied region.24 In recent years, by developing the health network system, prenatal care has progressed across the region of Babol; however, its effect on prenatal outcomes has not been evaluated. It is expected that the trend of stillbirths rate would decrease due to the promotion of prenatal care and family planning during the two recent decades; however, there is no evidence nor sufficient data on the trend of stillbirths and the associated risk factors for northern Iran. Since the rate of stillbirths essentially varies across different social, culture and racial status, the objective of this study was thus to investigate the trend of stillbirths rate and the corresponding risk factors in this region.
Methods
A retrospective study was conducted based on the data of hospital charts of two major Gynecological wards in Shahid Yahyanejat and Babol clinic hospitals in Babol, Northern Iran. This study was carried out in two phases, the first phase was an ecological study during the period of 1999-2008. The frequency of all livebirths and stillbirths were counted annually and the rate of stillbirths was calculated. In the second phase, a case control study of 150 cases of stillbirth, and 300 livebirths as controls, was conducted. A total of 150 stillbirths registered at the two major hospitals were recruited into the study from 2004 until 2008. For each case at each hospital and on the day that the stillbirth occurred, two controls of livebirths were selected randomly for the study. The data pertaining to maternal age, gestational age, gender of neonate, type of delivery, prior history of stillbirth, abortion, gravity, birth interval, diabetes mellitus and preeclampsia, as well as area of residence were extracted from the hospital records. Stillbirth was defined as fetal death at gestation of at least 20 weeks. The study was approved by the Research and Ethical Committee at Babol University of Medical Sciences.
Statistical analysis was performed using the SPSS software version 16.0. In bivariate analysis, the Chi-square test was used for categorical data and t-test for continuous data. In addition, the Chi-square test for trend was used to explore the declining trend of stillbirths rate over the study period. The logistic regression model was also used to estimate the unadjusted odds ratio and 95% confidence interval (95% CI) for each risk factor compared with the reference group. In logistic regression analysis both the likelihood ratio test was used to estimate single p-value for each independent variable regardless of the reference level and the Wald’s test p-value was also used for each category of exposure of interest compared with the reference group. The adjusted OR was calculated using the stepwise logistic regression model. In the multiple stepwise approach, the factors were included in the model using the forward method with entry and removal criteria of α=0.05 and α=0.10, respectively. In addition, for the purpose of efficiency of estimate of odds ratio for gestational age, we used the category of 34-37 weeks as the reference group. A p-value of <0.05 was considered as the level of significant.
Results
The results revealed that the highest rate of stillbirths (10.5 per 1000 deliveries) was in 1999 and the lowest (8.57 per 1000 deliveries) was observed in 2008 (Table 1). The trend of stillbirths rate was significantly declining during this period (p=0.001). (Fig. 1)
Table 1: The total number of births and the rate of stillbirths between 1999 and 2008.
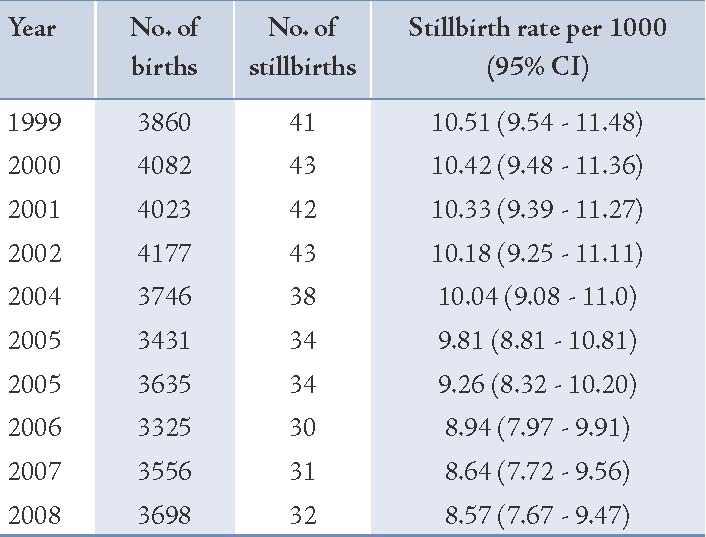
The mean age of mothers (SD) with stillbirths and livebirths were 26.2 ± 5.8 and 25.1 ± 5.3 years, respectively (p=0.04). The mean gestational age in the stillbirth group was significantly lower than the livebirth group (28.8 ± 5.8 vs. 38 ± 1.7; p=0.001). The majority of stillbirths were preterm and 46.7% of them were less than 28 weeks of gestational age. Table 2 shows that the frequency of stillbirths in women aged ≥35 years was higher than livebirths (10.7% vs. 5.7%), but the difference was not statistically significant. Roughly 90.7% of stillbirths were preterm and 72% were ≤33 weeks of gestation, while the majority of livebirths were ≥38 weeks of gestation (p=0.001). Somehow a similar percentage of stillbirths and livebirths at first gravity were 52.7% vs. 53.3%, while the percentage of gravity of ≥4 was higher in cases than in the controls. Both groups showed a similar distribution of rural residence area (76.7% vs. 80.7%; p=0.32). The frequency of male gender in the stillbirth group was not significantly found to be higher than in the controls (49.3% vs. 47%; p=0.64). About 4.7% of mothers with stillbirth had a history of diabetes compared with 3.7% of mothers with livebirths.
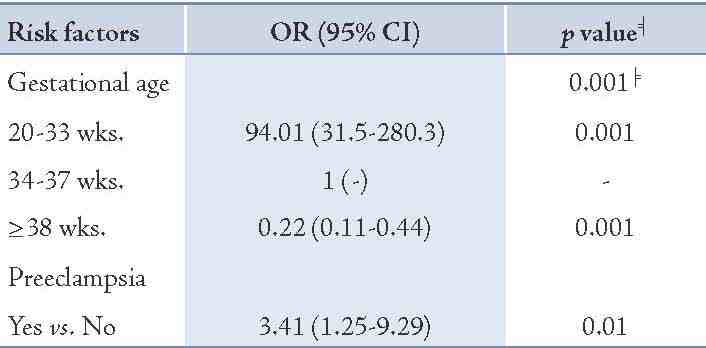
In addition, 8.7% of mothers with stillbirths had preeclampsia compared to 5.3% of mothers with livebirths. Moreover, 4% of mothers having stillbirth had prior history of stillbirths and 15.3% had a history of abortion, while these figures were 2.3% and 12.3%, respectively for the livebirth group (p=0.32 and p=0.37, respectively). The distribution of prenatal care was roughly similar between the two groups under study. Table 3 shows that gestational age and preeclampsia remained significant in the stepwise logistic regression model. By adjusting preeclampsia, very low gestational age (<34 weeks) had a very high risk of stillbirth (OR=94.01; p=0.001) compared with gestational age of 34-37 weeks as the reference category, and the risk diminished significantly (OR=0.22; p=0.001) for gestational age of ≥38 weeks. The association of preeclampsia after adjusting for gestational age remained significant (OR=3.41; p=0.01). Besides gestational age and preeclampsia in the model, other factors were not statistically significant.
Table 2: Distribution of risk factors for stillbirths, livebirths and the odds ratio (95% confidence interval).
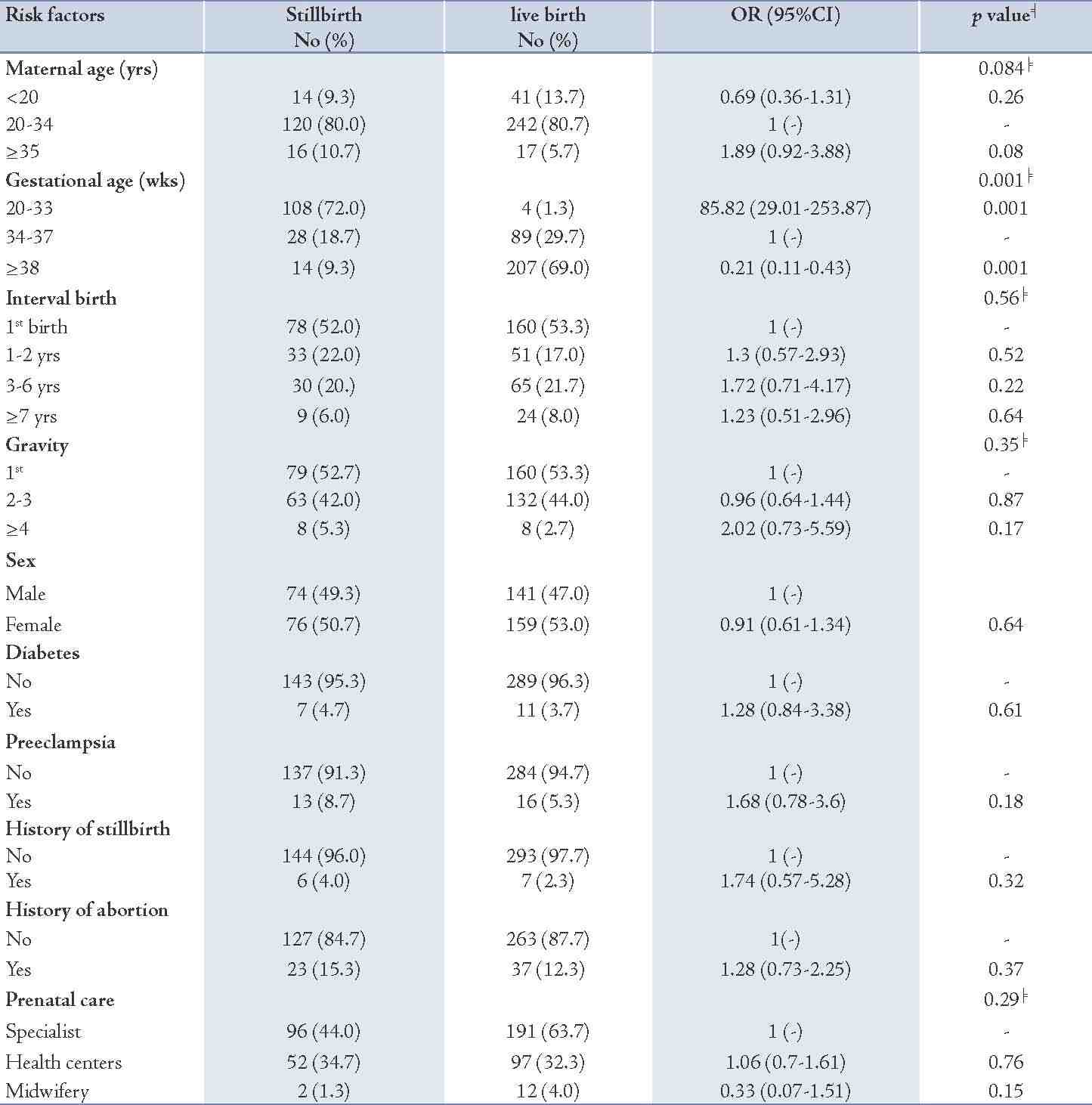
Figure 1: The trend of stillbirths rate during the study period (1999-2008) in Babol.
Table 3: The adjusted odds ratio of relevant risk factors remained significant in the stepwise logistic regression model.
Discussion
The findings from this study show that the rate of stillbirths has been declining from 10.51 in 1999 to 8.57 per 1000 deliveries (livebirths and stillbirths) in 2008. These figures are an indicator of promotion of the trend in prenatal care and coverage of health centers for family planning as well as the promotion of health condition of mothers, fetuses and infants. This decreasing trend may be attributed to interventional programs for controlling diabetes and preeclampsia and full coverage prenatal care in our health system in the recent decade. A similar finding of fetal death rate of 9.1 per 1000 births has been reported in the Dhahira region of Oman and 8.0 per 1000 births in Qatar, among our neighboring countries.13,25
In comparison with other reports, in a study by Macdorman et al. the rate of stillbirths in the United States was 6.41 and 6.2 per 1000 deliveries in 2002 and 2004, respectively.10 In another study by Gilbert et al. from Peru, the stillbirth rate was reportedly 6.1 per 1000 births.26 While in a study by Rahghozar et al. in Iran, the rate was 12.8 per 1000 deliveries.11 Another study in the province of Kerman (Rafsangan, southern Iran), it was 12.74 per 1000 deliveries in 2009.27 The highest rate of stillbirths (40 per 1000 deliveries) was reported in the province of Ahvaz, Iran in 2001 by Zareai.12 This province was essentially involved in war between Iran and Iraq before 1990. The northern part where the study took place was not affected directly as the southern portion of the country by the war. The relative low rate of stillbirths in the population under study may be explained by improvements in prenatal care in this defined catchment population with a relatively higher rate of human medical resources and maximum coverage by the health system. In addition, any changes in the population or access to healthcare during this decade might explain the decrease in the rate of stillbirths.
Our findings showed that a history of abortion and prior stillbirth was not significantly higher in women with stillbirths than those in the livebirths group. While in a study by Hadavi et al. a significant association was found between prior history of stillbirths with stillbirth.27 In another study conducted in Ahvaz, 80% of mothers with stillbirth had a prior history of stillbirth compared with 20% in the control group.12
Based on our findings, the mean age of mothers with stillbirth was significantly higher than those with livebirths and also the percentage of stillbirths among mothers aged >35 years was higher than the mother with livebirths. Our findings are consistent with a study by Ananth et al. in the United States,28 and by Nicolas et al. in Canada,26 which showed that women aged >35 years were at a greater risk of stillbirth. In a meta-analysis by Hung et al. 24 out of 31 cohort studies and all 6 case control studies showed an association between older age and stillbirth and the risk increased by 1.2 to 4.5 times in different studies.29 In another study conducted in Rafsanjan, southern Iran, a similar pattern of age was found in women with stillbirths and livebirths.27 The elevated risk may be partially explained by the relationship between increasing age with higher rate of abortion, prior stillbirth, diabetes, hypertension, multiparity and declomentation, which are accompanied with increasing risk of stillbirth.
In terms of gestational age, we found that the mean gestational age was significantly lower in the stillbirth group than the controls and most of the stillbirths were preterm, 46.7% of them were at less than 28 weeks of gestation. Although low gestational age was more common among the stillbirth than the live birth group, gestational age itself is not a risk factor for stillbirth but weak fetuses tend to get out earlier than healthy fetuses. Nevertheless, our results were similar to those reported by Goldenberg et al. in the USA.30 Another study in Ahvaz, showed a similar pattern for the mean age of gestation which was reported for stillbirths and livebirths.12 Our results are inconsistent with the study by Jehan et al. in Pakistan which showed that 51% of stillbirths occurred at GA >37 weeks.31 The pattern and evaluation of GA might be different in some developing countries such as Pakistan compared to Iran.
In our results, the frequency of male and female sexes between the cases and the controls was not significant, this finding corresponds with the study by Zarei et al.12 and Shahghibi et al.32 in Iran which reported that intrauterine growth retardation (IUGR) was not associated with gender, while a study conducted in Shiraz, Iran by Jahanfar reported an association between male gender and stillbirths.33
In our results, the percentage of first gravity was similar between the case group and the control group but a greater proportion of stillbirths was found at forth gravity or higher compared with the controls. In a study by Jammeh et al. in Gambia, stillbirth was also significantly associated with fourth gravity or higher.34 In addition, our findings showed that a higher percentage of women among the case group had a history of diabetes compared with the controls but the difference was not statistically significant. Other studies have also reported that among women with overt diabetes, high glucose level increased the risk of fetus disorders and IUGR and nonspecific causes of stillbirth among overt diabetes.35 In our study, 4.7% of the case group had a history of diabetes vs. 3.7% among the controls. While in a study by Shahghibi et al. in Iran, 1.2% of stillbirths was attributed to diabetes mellitus.32 In another study by Siver et al. in USA, the risk of stillbirth was elevated significantly among diabetic women and about 4.4% of overt diabetes was attributed to stillbirths, which is roughly similar to our findings.36 Moreover, in our findings, preeclampsia significantly increased the risk of stillbirth up to 3.41 times. Another study conducted in Western Iran, also reported that cases had 12% of stillbirth preeclampsia,32 which is slightly higher than ours. With this regard, our results are also consistent with those reported by Jehan et al.29 in Pakistan and Wilson et al. in the USA.37
In this current study, no significant difference was observed in terms os obstetric prenatal care between the cases and the controls. Published data reported that sufficient visits and prenatal care significantly reduce the risk of stillbirth. One possible explanation for the lack of significant findings might be due to an undetermined data of the number of prenatal care visits in hospital records in our study.
Our study had several advantages. First, this study was conducted in a rather homogenous population with respect to culture, racial and religious status, which was covered by the health network system with roughly similar prenatal care and all deliveries of the study population occured in hospitals. Second, the two major hospitals in which the cases and controls were recruited, cover roughly 95% of all birth deliveries in the study population. Third, all the charts of fetus death were reviewed and all charts included sonography in order to measure the gestational age; besides fetus death at <20 weeks gestational age were excluded.
This study may have had some limitations. First, the findings of association with preterm delivery and gestational age were likely due to the way the controls were selected. In fact, the controls were selected from livebirths on the same day stillbirths were born. This may likely have caused a bias towards these findings. A prospective cohort study is required to establish such association. Second, we were not able to assess the effect of other risk factors such as smoking, addiction and obesity. Based on our culture, the rates of smokers and addicted women are very low and thus they were not sufficiently presentated in our study samples. Third, since the nature of the study was retrospective based on hospital charts, the data of some risk factors such as obesity was not registered in the hospital charts. Furthermore, the estimates drawn from two hospitals under study may not represent the actual rate of stillbirths in the study region. The proportion of stillbirths and livebirths which occurred at home or anywhere outside of these two hospitals during the study period of 1999-2008 was not included in the study. However, this proportion would not have exceeded 10%.
Conclusion
Overall, preterm delivery and preeclampsia have a significantly positive association with the risk of stillbirth. For greater impact of prevention of this adverse outcome, it is necessary to prevent high risk pregnancy in women aged ≥35 years and to promote the extension of prenatal care to control diabetes and preeclampsia. Thus, intervention through educational programs should primarily focus towards high risk groups in order to further reduce the risk of stillbirth.
Acknowledgements
The authors reported no conflict of interests and no funding was received for this work.
References
1. World Health Organization. definitions and indicators in family planning, maternal and child health and reproductive health. Geneva, WHO press, 2001; 450:826-34.
2. Goldenberg RL, McClure EM, Saleem S, Reddy UM. Infection-related stillbirths. Lancet 2010 Apr;375(9724):1482-1490.
3. Sachs BP, Fretts RC, Gardner R, Hellerstein S, Wampler NS, Wise PH. The impact of extreme prematurity and congenital anomalies on the interpretation of international comparisons of infant mortality. Obstet Gynecol 1995 Jun;85(6):941-946.
4. Wilson AL, Fenton LJ, Munson DP. State reporting of live births of newborns weighing less than 500 grams: impact on neonatal mortality rates. Pediatrics 1986 Nov;78(5):850-854.
5. Bernabé-Ortiz A, White PJ, Carcamo CP, Hughes JP, Gonzales MA, Garcia PJ, et al. Clandestine induced abortion: prevalence, incidence and risk factors among women in a Latin American country. CMAJ 2009 Feb;180(3):298-304.
6. Centers for Disease Control and Prevention (CDC). stillbirths, http://www.cdc.gov/ncbddd/bd/stillbirths.htm. Retrieved 3/5/2009.
7. McClure EM, Saleem S, Pasha O, Goldenberg RL. Stillbirth in developing countries: a review of causes, risk factors and preventive strategies. Int Matern Fetal Neonatal Med. 2009;22(3):183-190 .
8. Stanton C, Lawn JE, Rahman H, Wilczynska-Ketende K, Hill K. Stillbirth rates: delivering estimates in 190 countries. Lancet 2006 May;367(9521):1487-1494.
9. Hamilton BE, Miniño AM, Martin JA, Kochanek KD, Strobino DM, Guyer B. Annual summary of vital statistics: 2005. Pediatrics 2007 Feb;119(2):345-360.
10. MacDorman MF, Kirmeyer S. Fetal and perinatal mortality, United States, 2005. Natl Vital Stat Rep 2009 Jan;57(8):1-19.
11. Rahghozar M. Kazem-Mohammad, Ramazani-Tehrani F. Trend of still birth in Iranian women aged 15-49 years during forth decades: 1956-1996. Hakim Res J 2001;4(2):85-91. in Persian.
12. Zarei R. Frequency of Intra utrin death and the associated factors in women referred ti Ahvaz Emam Khomini hospital. Ahvaz Uni Med Sci J 2009;8(4):437-443. in Persian.
13. Salameh K, Rahman S, Al-Rifai H, Masoud A, Lutfi S, Abdouh G, et al. An analytic study of the trends in perinatal and neonatal mortality rates in the State of Qatar over a 30-year period (1977 to 2007): a comparative study with regional and developed countries. J Perinatol 2009 Nov;29(11):765-770.
14. Guidotti RJ, Kandasamy T, Betrán AP, Merialdi M, Hakimi F, Van Look P, et al. Monitoring perinatal outcomes in hospitals in Kabul, Afghanistan: The first step of a quality assurance process. J Matern Fetal Neonatal Med 2009 Apr;22(4):285-292.
15. Ngoc NT, Merialdi M, Abdel-Aleem H, Carroli G, Purwar M, Zavaleta N, et al. Causes of stillbirths and early neonatal deaths: data from 7993 pregnancies in six developing countries. Bull World Health Organ 2006 Sep;84(9):699-705.
16. Sørnes T. Umbilical cord knots. Acta Obstet Gynecol Scand 2000 Mar;79(3):157-159.
17. Carey JC, Rayburn WF. Nuchal cord encirclements and risk of stillbirth. Int J Gynaecol Obstet 2000 May;69(2):173-174.
18. McClure EM, Saleem S, Pasha O, Goldenberg RL. Stillbirth in developing countries: a review of causes, risk factors and prevention strategies. J Matern Fetal Neonatal Med 2009 Mar;22(3):183-190.
19. Healy AJ, Malone FD, Sullivan LM, Porter TF, Luthy DA, Comstock CH, et al; FASTER Trial Research Consortium. Early access to prenatal care: implications for racial disparity in perinatal mortality. Obstet Gynecol 2006 Mar;107(3):625-631.
20. Willinger M, Ko CM, Reddy UM. Racial disparity in stillbirth risk across gestation in the United States. Am J Obstet Gynecol. 2009; 201(5):469.e1-8.
21. Stephansson O, Dickman PW, Johansson A, Cnattingius S. Maternal hemoglobin concentration during pregnancy and risk of stillbirth. Am J Obstet Gynecol 2001;248(2):463-469 .
22. Salihu HM, Kinniburgh BA, Aliyu MH, Kirby RS, Alexander GR. Racial disparity in stillbirth among singleton, twin, and triplet gestations in the United States. Obstet Gynecol 2004 Oct;104(4):734-740.
23. Clapp JF III, Stepanchak W, Hashimoto K, Ehrenberg H, Lopez B. The natural history of antenatal nuchal cords. Am J Obstet Gynecol 2003 Aug;189(2):488-493.
24. Ministry of Health and Medical Education. Islamic Republic of Iran, Social determinants of health in the Islamic Republic of Iran, prepared with the technical assistance of WHO, 2007, p. 24. http://www.behdasht.gov.ir/uploads 291_1041_SDH.
25. Patel PK. Profile of fetal deaths in dhahira region, oman. Oman Med J 2008 Jan;23(1):28-31.
26. Gilbert NL, Casapía M, Joseph SA, Ryan JA, Gyorkos TW. Inadequate prenatal care and the risk of stillbirth in the Peruvian Amazon. Int J Gynaecol Obstet 2010 May;109(2):155-156.
27. Hadavi M, Alidallaki S, Abedini M. Determinants of prenatal mortality in Rafasanghan during 2004-2006. Rafsajan Uni Med Sci J 2009;8(2):117-128. in Persian.
28. Ananth CV, Liu S, Kinzler WL, Kramer MS. Stillbirths in the United States, 1981-2000: an age, period, and cohort analysis. Am J Public Health 2005 Dec;95(12):2213-2217.
29. Huang L, Sauve R, Birkett N, Fergusson D, van Walraven C. Maternal age and risk of stillbirth: a systematic review. CMAJ 2008 Jan;178(2):165-172.
30. Goldenberg RL, Foster JM, Cutter GR, Nelson KG, Nelson KG. Fetal deaths in Alabama, 1974-1983: a birth weight-specific analysis. Obstet Gynecol 1987 Dec;70(6):831-835.
31. Jehan I, McClure EM, Salat S, Rizvi S, Pasha O, Harris H, et al. Stillbirths in an urban community in Pakistan. Am J Obstet Gynecol 2007;197(3):257. e1-8.
32. Shahghibi S, Ghadami N. S study of still birth rate in Rasol hospital in Sanandagh, Iran, 1995-96. Kurdestan Uni Med Sci J 1998;2(7):16-20. in Persian.
33. Jahanfar SH, Ghiyasi P, Haghani H. Risk factor related intrauterine fetal death in Iran. Shiraz Med. J 2005;6(3):1-14.
34. Jammeh A, Vangen S, Sundby J. Stillbirths in rural hospitals in the Gambia: a cross-sectional retrospective study. Int J Obstet Gynecol, 2010; doi:10.1155/2010/186867
35. Fretts RC. Etiology and management of antepartum fetal death. 2008; available at:url:http://www.uptodate.com.
36. Silver RM, Varner MW, Reddy U, Goldenberg R, Pinar H, Conway D, et al. Work-up of stillbirth: a review of the evidence. Am J Obstet Gynecol 2007 May;196(5):433-444.
37. Wilson RE, Alio AP, Kirby RS, Salihu HM. Young maternal age and risk of intrapartum stillbirth. Arch Gynecol Obstet 2008 Sep;278(3):231-236.
|