Cystic fibrosis (CF) is one of the most common autosomal recessive disorders, affecting approximately 1 in every 2000 live births. It is more frequently observed among the Caucasian and Northern European descent populations.1 In Oman, the estimated prevalence of CF is 10.3 per 100 000 individuals. The most common transmembrane conductance regulator (CFTR) genotypes in Oman are p.Ser549Arg and DelF508, with prevalences of 51.9% and 12.3%, respectively.2
CF is a multisystemic disease that affects multiple organs. Progressive decline in lung function is the primary cause of morbidity and mortality in this population.3 This decline is related to chronic airway infection and recurrent pulmonary exacerbations.4,5 The microorganism causing this chronic infection and its resistance pattern play a significant role in the trajectory of lung function decline.6,7 Routine sampling to obtain respiratory cultures at every clinic visit–even in the absence of respiratory symptoms–and during CF exacerbations is recommended. Sputum is the preferred method; however, in patients who are unable to expectorate, such as young children, cough or throat swabs, and oropharyngeal suction are acceptable alternatives and have been shown to correlate with lower airway sampling in CF. This routine surveillance can guide proactive eradication for certain bacteria like Pseudomonas aeruginosa, the antibiotic choice during exacerbation, and helps in infection control measures.8
P. aeruginosa is a common pathogen in CF and has been associated with reduced life expectancy in this population.9 In the Middle East, it has been noted that CF patients tend to get earlier growth of P. aeruginosa from respiratory cultures compared to international data. A study conducted in Saudi Arabia found that P. aeruginosa was the most common organism in first-taken culture from CF children (44%), followed by Haemophilus influenza (16%) and Methicillin-Susceptible Staphylococcus aureus (MSSA) (15%).10 Several risk factors for early P. aeruginosa acquisition have been reported in the literature, including female sex, CFTR genotype, and coinfection with MSSA.11,12 A recent study showed that a prior infection with any bacterial pathogen increases the risk of P. aeruginosa acquisition with a 16% increase for every additional pathogen.13
Methicillin-resistant S. aureus (MRSA) is another emerging challenge for patients with CF, which is associated with reduced survival.14 Several risk factors for persistent MRSA have been identified, including pancreatic insufficiency status, CF-related diabetes, hospitalization rate, co-infection with P. aeruginosa, and receiving care at a center with a higher prevalence of MRSA infection.15
To the best of our knowledge, no studies have investigated the microbiological pattern of chronic airway infections in CF patients in Oman. This study aimed to assess the pattern of airway bacterial growth among different age groups of CF patients in Oman. It also aimed to identify possible risk factors for the hypothesized early P. aeruginosa acquisition in this population. This would guide CF healthcare providers in Oman in their preventive and treatment strategies, and may encourage further prospective and environmental studies to identify modifiable risk factors for early P. aeruginosa infection.
Methods
This retrospective single-center cross-sectional study included all patients who attended the CF clinic at Royal Hospital, Oman between January 2004 and December 2020. Royal Hospital is one of two CF centers in the country. The 16-year period was chosen based on the availability of electronic data records. The study was approved by the Ministry of Health Centre of Studies and Research (Ethical approval Number SRC#67/2020).
In this study, the diagnosis of CF was based on international criteria, which requires the presence of at least one typical phenotypic feature (e.g., chronic pulmonary disease, chronic sinusitis, characteristic gastrointestinal and nutritional abnormalities, salt loss syndrome), a history of CF in a sibling, or a positive newborn screening test along with one of the following: a positive sweat chloride test, two CFTR mutation known to cause CF on separate alleles, or an abnormality in nasal potential difference testing.16
Demographic information (including current age, sex, date of birth, and geographic region), date of CF diagnosis, genotype, and number of siblings diagnosed with CF were retrieved from the electronic health records (Al-Shifa system). The dates and results of all respiratory cultures, including cough swabs, nasopharyngeal aspirate, bronchoalveolar lavage, and sputum cultures, were obtained to capture all airway sampling methods used by the treating team. Additionally, hospitalizations dates related to CF exacerbation and the antibiotics used during these admissions were collected. Age at first acquisition of different microorganisms was calculated from date of birth and date of positive culture. Multi-drug resistant P. aeruginosa (MDRO) is defined as P. aeruginosa not susceptible to ≥ 1 agent in ≥ 3 classes of antimicrobials. Participants were divided into two groups based on the age of the first P. aeruginosa positive culture: early acquisition, defined as the first positive culture of P. aeruginosa before the age of two years, and the late acquisition. The two groups were compared in terms of sex, CFTR mutation, geographic area, and presence of a sibling with CF.
The data were analyzed using SPSS Statistics (IBM Corp. Released 2023. IBM SPSS Statistics for Windows, Version 29.0.2.0 Armonk, NY: IBM Corp.). Categorical variables were described using frequencies and percentages. Relationships between the parametric variables were analyzed using the chi-square test and t-test, while non-parametric variables were assessed using the Mann-Whitney U test for pairwise comparisons between two independent groups and the Kruskal-Wallis test to evaluate differences among three or more independent groups. A p-value of < 0.05 was considered statistically significant.
Results
The study included 114 patients, with 60.5% being male. The majority of patients were homozygous p.Ser549Arg (60.5%) and most commonly originated from the North Al Batinah governorate (45.6%) [Table 1].
Table 1: Patients’ demographics.
|
Sex
|
|
|
Male
|
69 (60.5)
|
|
Female
|
45 (39.5)
|
|
Governorate
|
|
|
Al Dhakiliah
|
20 (17.5)
|
|
North Al Batinah
|
52 (45.6)
|
|
Al Dhahira
|
15 (13.2)
|
|
Al Buraimi
|
5 (4.4)
|
|
South Al Batinah
|
15 (13.2)
|
|
Muscat
|
5 (4.4)
|
|
South A'Sharqiyah
|
1 (0.9)
|
|
Dhofar
|
1 (0.9)
|
|
Siblings with cystic fibrosis
|
|
|
0
|
68 (59.6)
|
|
≥ 1
|
46 (40.4)
|
|
CFTR genotypes*
|
|
|
Homozygous p.Ser549Arg
|
69 (60.5)
|
|
Homozygous DelF508
|
12 (10.5)
|
|
Other heterozygous
|
16 (14.0)
|
*Cystic fibrosis transmembrane conductance regulator (CFTR) genotype is missing for six patients.
A total of 2393 positive bacterial cultures were studied. Sampling methods included sputum (n = 1533, 66.6%) and cough swab culture (n = 768, 33.4%). Among all positive bacterial cultures, P. aeruginosa accounted for 713 cultures (43.3%), followed by MSSA (15%) and H. influenza/parainfluenza (8.5%). MRSA accounted for 5.7% of positive cultures, while MDR P. aeruginosa accounted for 3.7%. Klebsiella pneumonia and Stenotrophomonas maltophilia accounted for 3.1% and 1.3% of cultures, respectively. P. aeruginosa was the most common organism across all age groups, accounting for 30.4% of all respiratory cultures obtained before the age of one year, which peaked up to 58.2% between two and three years of age. Half of the studied population were P. aeruginosa positive by 29 months of age. Twenty-six (22.8%) patients were positive for P. aeruginosa on their first respiratory culture, while 56 (49.1%) patients had three or more positive respiratory cultures for P. aeruginosa. MRSA accounted for 7.2% of all positive cultures under the age of one year and peaked at 14.8% between the ages of four and five years [Figure 1].
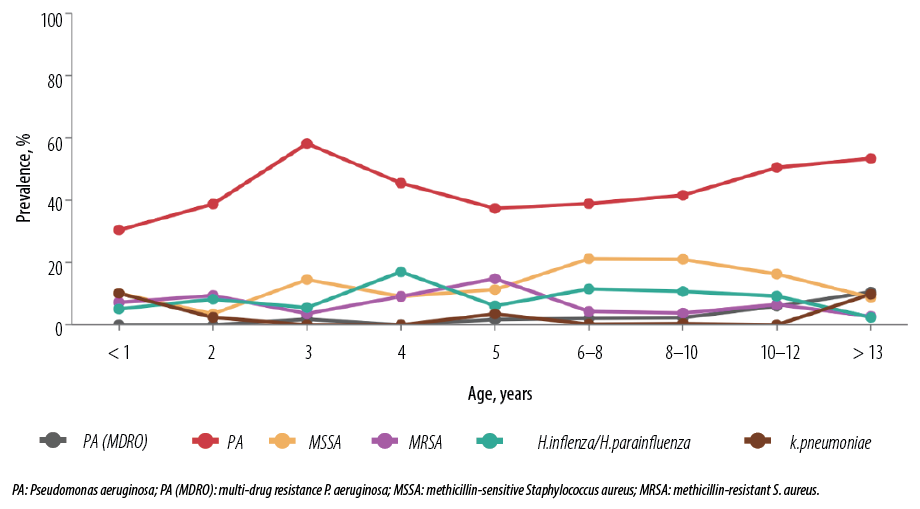 Figure 1: Prevalence of bacterial culture distributed based on age group.
Figure 1: Prevalence of bacterial culture distributed based on age group.
Patients were divided into two groups based on the age at which they first acquired P. aeruginosa: ≤ 24 months (early acquisition) and > 24 months old (late acquisition). Of all studied patients, 40 (47.6%) had early P. aeruginosa acquisition, while 44 (53.4%) had a late acquisition.
The hypothesized risk factors for early P. aeruginosa acquisition, including sex, governerate where the patient came from, CF genotype, and the presence of one or more siblings with CF, were studied. Male sex showed a statistically significant association with earlier P. aeruginosa acquisition. None of the other risk factors showed a statistically significant association with early P. aeruginosa acquisition [Table 2].
Table 2: Risk factors compared between early P. aeruginosa acquisition (defined by a positive respiratory culture for PA before the age of two years) and late acquisition groups.
|
Number (%)
|
40 (47.6)
|
44 (52.4)
|
|
|
Sex
|
|
|
|
|
Male
|
29 (72.5)
|
23 (52.3)
|
0.03
|
|
Female
|
11 (27.5)
|
21 (47.7)
|
|
Governorate
|
|
|
|
|
A'Dakhiliah
|
7 (17.5)
|
8 (18.2)
|
0.41
|
|
North Al Batinah
|
18 (45.0)
|
21 (47.7)
|
|
A'Dhahirah
|
8 (20.0)
|
3 (6.8)
|
|
Al Buraimi
|
2 (5.0)
|
1 (2.3)
|
|
South Al Batinah
|
4 (10.0)
|
9 (20.5)
|
|
Muscat
|
1 (2.5)
|
2 (4.5)
|
|
Siblings with cystic fibrosis
|
|
|
|
|
0
|
24 (60.0)
|
29 (65.9)
|
0.57
|
|
≥ 1
|
16 (40.0)
|
15 (34.1)
|
|
CFTR genotypes*
|
|
|
|
|
Homozygous p.Ser549Arg
|
26 (65.0)
|
32 (76.2)
|
|
Homozygous DelF508
|
4 (10.0)
|
5 (11.9)
|
|
Other heterozygous
|
4 (10.0)
|
1 (2.4)
|
*Cystic fibrosis transmembrane conductance regulator (CFTR) genotype is missing for two patients in the late group.
Discussion
Our study demonstrated that patients with CF in Oman acquire P. aeruginosa at an early age. It also showed that P. aeruginosa is the most common organism isolated from respiratory cultures across all age groups in CF patients. This contrasts with the international data. According to the 2021 Cystic Fibrosis Foundation Patient Registry (CFFPR) report, MSSA was the most common organism among all pediatric age groups.17 Similarly, the Australian Cystic Fibrosis Data Registry showed a predominance of MSSA in children under seven years of age (22%), followed by H. influenzae (20%).18 A study conducted in Spain showed that MSSA was the most common infection in young patients between the ages of 6–10 years, while P. aeruginosa infections became more prevalent in the late adolescent age group.19 Another study from the US reported that H. influenzae was the most prevalent organism to grow from respiratory cultures in children under two years old.20 Additionally, a population-based study in the US showed that the overall incidence of P. aeruginosa was lower in 2020 (36%) compared to 2018 (51%), suggesting a declining trend.7 While our findings differ from the international data, they are consistent with data from the region. A study from Saudi Arabia showed that CF patients tend to have early P. aeruginosa infection and it was the most prevalent bacterial culture.10 Another follow-up study conducted in Saudi Arabia revealed an increase in the prevalence of P. aeruginosa from 34% to 53% and a decrease in the prevalence of MSSA and H. influenza over a seven-year period.21
MDR P. aeruginosa, defined as P. aeruginosa that is not susceptible to ≥ 1 agent in ≥ 3 classes of antimicrobials, is an emergent challenge among the CF population. In our study, it was more prevalent in patients ≥ 13 years (10.5%). Among all positive cultures, MDR P. aeruginosa accounted for 3.7%, which is comparable to the 3.5% reported in the CFFPR-2021.17
We observed a peak of MRSA at five years old (14.8%). In contrast, the CFFPR-2021 report showed the highest prevalence of MRSA in individuals aged 10–20 years, accounting for approximately 20% of positive cultures.17 Similarly, regional data from Saudi Arabia showed an MRSA prevalence of 11% among CF patients, which was acquired at a mean age of 10.4 ± 7.2 years. That study also noted an increasing trend in MRSA infection with 79% of positive cultures occurring between 2010–2016 compared to 26% between 2002–2009.22
Several risk factors for early P. aeruginosa acquisition have been reported in the literature. A study in the USA by Maselli et al,11 showed an association between early P. aeruginosa acquisition and female sex, homozygous deltaF508 mutation, co-infection with MSSA, and length of hospital stay. In our study, male patients had early P. aeruginosa acquisition. There was no statistically significant difference in CFTR genotype between the early and late P. aeruginosa groups.
In a study examining the potential role of social interaction as a risk factor for early P. aeruginosa infection, the interaction between a younger patient and an older CF patient was identified a risk factor.16 Other studies showed that CF siblings can have P. aeruginosa cross-infection.23,24 In our study, the presence of a sibling with CF was not a statistically significant risk factor for early acquisition.
Few environmental factors have been studied previously to address the causes of P. aeruginosa infections. The Morbidity and Mortality Weekly Report of the Centers for Disease Control and Prevention surveillance for waterborne disease outbreaks in the US (1993–1994) revealed that water sources, such as hot tubs and swimming pools, are sources of P. aeruginosa infections.25 Another study showed that jacuzzis and hot tubs had the highest sources of P. aeruginosa infections among the studied water sources.26 These parameters could not be studied in our study, but there was no relationship between demographic region and early acquisition.
In an adult study, it was noted that in patients with chronic obstructive pulmonary disease, pathogenic bacteria were isolated from the nebulization sets, which includes P. aeruginosa along with other pathogens.27 This is a possible risk factor in our population; however, due to the retrospective nature of our study, it was not possible to retrieve such data.
There are several limitations to our study including its retrospective design leading to the risk of missing data. Additionally, we did not control for variations in airway sampling methods. Some of the possible risk factors, such as healthcare facility visits before diagnosis or water contamination, could not be studied. We recommend future prospective studies in Oman addressing these limitations. These studies should focus on healthcare exposures before CF diagnosis, including receiving nebulized medications, co-infection within the same institution, and environmental surveillance.
Conclusion
Our data demonstrated an early acquisition of P. aeruginosa and its predominance across all age groups. Among the studied risk factors, only the male sex showed a significant association with early acquisition. Early lower airway sampling with bronchoalveolar lavage for early P. aeruginosa detection and eradication could be considered by CF healthcare providers. Further studies are needed to investigate the environmental risk factors for early P. aeruginosa acquisition in Oman.
Acknowledgment
We would like to thank Mr. Sathiya Murthy for his assistance in data analysis.
Disclosure
The authors declare no conflicts of interest. No funding was received for this study.
references
- 1. Cystic fibrosis foundation. Annual report. [cited 2022 Sep 10]. Available from: https://www.cff.org/about-us/annual-report.
- 2. Al Oraimi S, Al Shidhani K, Al Harthi H, Al Sinani S, Al Busaidi N, Al Bimani M, et al. Prevalence and characteristics of cystic fibrosis in Omani children: a multi-center cross-sectional study. Oman Med J 2022 Nov;37(6):e444.
- 3. Grasemann H, Wiesemann HG, Ratjen F. [The importance of lung function as a predictor of 2-year mortality in mucoviscidosis]. Pneumologie 1995 Aug;49(8):466-469.
- 4. Pillarisetti N, Williamson E, Linnane B, Skoric B, Robertson CF, Robinson P, et al; Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF). Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med 2011 Jul;184(1):75-81.
- 5. Pamukcu A, Bush A, Buchdahl R. Effects of pseudomonas aeruginosa colonization on lung function and anthropometric variables in children with cystic fibrosis. Pediatr Pulmonol 1995 Jan;19(1):10-15.
- 6. Hahn A, Burrell A, Fanous H, Chaney H, Sami I, Perez GF, et al. Antibiotic multidrug resistance in the cystic fibrosis airway microbiome is associated with decreased diversity. Heliyon 2018 Sep;4(9):e00795.
- 7. Crull MR, Somayaji R, Ramos KJ, Caldwell E, Mayer-Hamblett N, Aitken ML, et al. Changing rates of chronic pseudomonas aeruginosa infections in cystic fibrosis: a population-based cohort study. Clin Infect Dis 2018 Sep;67(7):1089-1095.
- 8. Burgel PR, Southern KW, Addy C, Battezzati A, Berry C, Bouchara JP, et al. Standards for the care of people with cystic fibrosis (CF); recognising and addressing CF health issues. J Cyst Fibros 2024 Mar;23(2):187-202.
- 9. Durda-Masny M, Goździk-Spychalska J, John A, Czaiński W, Stróżewska W, Pawłowska N, et al. The determinants of survival among adults with cystic fibrosis-a cohort study. J Physiol Anthropol 2021 Nov;40(1):19.
- 10. Hanaa HB. Microbiological data of cystic fibrosis patients in a tertiary care center in Saudi Arabia. KMJ-Kuwait Medical Journal 2004;36(3):177-181.
- 11. Maselli JH, Sontag MK, Norris JM, MacKenzie T, Wagener JS, Accurso FJ. Risk factors for initial acquisition of Pseudomonas aeruginosa in children with cystic fibrosis identified by newborn screening. Pediatr Pulmonol 2003 Apr;35(4):257-262.
- 12. Rosenfeld M, Emerson J, McNamara S, Thompson V, Ramsey BW, Morgan W, et al; EPIC Study Group. Risk factors for age at initial Pseudomonas acquisition in the cystic fibrosis epic observational cohort. J Cyst Fibros 2012 Sep;11(5):446-453.
- 13. Mésinèle J, Ruffin M, Guillot L, Boëlle PY, Corvol H. Airway infections as a risk factor for Pseudomonas aeruginosa acquisition and chronic colonisation in children with cystic fibrosis. J Cyst Fibros 2023 Sep;22(5):901-908.
- 14. Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA 2010 Jun;303(23):2386-2392.
- 15. Jennings MT, Dasenbrook EC, Lechtzin N, Boyle MP, Merlo CA. Risk factors for persistent methicillin-resistant Staphylococcus aureus infection in cystic fibrosis. J Cyst Fibros 2017 Nov;16(6):681-686.
- 16. Farrell PM, White TB, Ren CL, Hempstead SE, Accurso F, Derichs N, et al. Diagnosis of cystic fibrosis: consensus guidelines from the cystic fibrosis foundation. J Pediatr 2017 Feb;181:S4-S15.e1.
- 17. Cystic fibrosis foundation. Patient registry. [cited 2023 Jan 26]. Available from: https://www.cff.org/medical-professionals/patient-registry.
- 18. Cystic fibrosis south Australia. 2020 Australian cystic fibrosis data registry annual report. 2021 [cited 2023 Jan 26]. Available from: https://www.cfsa.org.au/acfdr-2020-annual-report/.
- 19. de Dios Caballero J, Del Campo R, Royuela A, Solé A, Máiz L, Olveira C, et al; GEIFQ (Grupo Español para el Estudio de la Colonización/Infección Broncopulmonar en Fibrosis Quística). Bronchopulmonary infection-colonization patterns in Spanish cystic fibrosis patients: Results from a national multicenter study. J Cyst Fibros 2016 May;15(3):357-365.
- 20. Rosenfeld M, Gibson RL, McNamara S, Emerson J, Burns JL, Castile R, et al. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol 2001 Nov;32(5):356-366.
- 21. Banjar H, Ghawi A, AlMogarri I, Alhaider S, Alomran H, Hejazi A, et al. First report on the prevalence of bacteria in cystic fibrosis patients (CF) in a tertiary care center in Saudi Arabia. Int J Pediatr Adolesc Med 2022 Jun;9(2):108-112.
- 22. Banjar H, Al-Qahtani H, Yasin W, Al-Wgait W, Al-Amer H, Raja R, et al. The first report of Methicillin-resistant Staphylococcus aureus (MRSA) in cystic fibrosis (CF) patients in Saudi Arabia. Int J Pediatr Adolesc Med 2020 Dec;7(4):186-190.
- 23. Renders NH, Sijmons MA, van Belkum A, Overbeek SE, Mouton JW, Verbrugh HA. Exchange of Pseudomonas aeruginosa strains among cystic fibrosis siblings. Res Microbiol 1997 Jun;148(5):447-454.
- 24. Tubbs D, Lenney W, Alcock P, Campbell CA, Gray J, Pantin C. Pseudomonas aeruginosa in cystic fibrosis: cross-infection and the need for segregation. Respir Med 2001 Feb;95(2):147-152.
- 25. Centers for Disease Control and Prevention. Surveillance for waterborne-disease outbreaks--United States, 1993-1994. 1996 [cited 2023 Jan 30]. Available from: https://stacks.cdc.gov/view/cdc/26708.
- 26. Caskey S, Stirling J, Moore JE, Rendall JC. Occurrence of Pseudomonas aeruginosa in waters: implications for patients with cystic fibrosis (CF). Lett Appl Microbiol 2018 Jun;66(6):537-541.
- 27. Jarvis S, Ind PW, Thomas C, Goonesekera S, Haffenden R, Abdolrasouli A, et al. Microbial contamination of domiciliary nebulisers and clinical implications in chronic obstructive pulmonary disease. BMJ Open Respir Res 2014 Feb;1(1):e000018.