Zoledronic acid, a third-generation bisphosphonate, is routinely prescribed for post-menopausal osteoporosis and is generally administered intravenously. The drug is also used to manage malignancy-associated hypercalcemia, multiple myeloma, secondary bone metastasis, Paget’s disease, and other causes of decreased bone mineralization.1 Zoledronic acid works by promoting osteoclast apoptosis, thereby preventing bone reabsorption and reducing the likelihood of hip fractures.2,3
Zoledronic acid infusion is usually well-tolerated in most patients with osteoporosis. Most frequently reported adverse symptoms are fever, arthralgia, myalgia, influenza-like symptoms, and musculoskeletal pain. Although adverse reactions to the first dose occur in 10–30% of cases, their recurrence will generally decrease in severity following subsequent infusions.4 These adverse events tend to be self-limiting and resolve within days.
Other rare side effects that are generally associated with cancer patients include jaw bone osteonecrosis, femur neck bone fracture, arrhythmia, severe eye inflammation, electrolyte abnormalities, and renal impairment.3 In this case report, we describe a case of flare-up of osteoarthritis in a woman treated with zoledronic acid for osteoporosis.
Case Report
The patient was a 62-year-old woman, known to have osteoarthritis, osteoporosis, and peptic ulcer disease. Detailed history revealed menopause as the only strong risk factor associated with her osteoporosis. She had no history of long-term steroid intake or hormonal replacement therapy. There was no family history of hip fractures. She was taking calcium 600 mg and vitamin D 800 IU supplements once daily, and when necessary, paracetamol. Her previous examinations and radiological investigations had shown features of osteoarthritis in bilateral knees and in the small joints of her hands with Heberden and Bouchard nodules.
Dual-energy X-ray absorptiometry results (T score = -3.5) confirmed the diagnosis of osteoporosis. The patient gave no previous history of treatment with bisphosphonates. Upon confirmation of osteoporosis, zoledronic acid 5 mg intravenous IV infusion was given at a constant rate, which was completed uneventfully. The patient's hydration status, renal function, serum vitamin D, and calcium were all within normal ranges.
About 12 hours after the zoledronic acid infusion, the patient developed fever with chills, generalized body aches, and severe low back pain. Paracetamol was given, and the fever and back pain subsided within a day. She then developed bilateral knee pain with swelling, which resolved within two to three days. Three days post-infusion, she started to have severe pain in the bilateral wrists and small joints of the hands (proximal and distal interphalangeal joints). The pain was severe and worsened with time, and was associated with swelling, redness, and restricted joint movement. The pain score was estimated at 9/10.
Bilateral hand examination revealed tenderness over the Heberden and Bouchard nodes and restricted and painful hand movements [Figure 1]. On wrist examination, swelling over the medial carpometacarpal joints, severe tenderness, and restriction of movements were noted. Joint effusion was present in both knees.
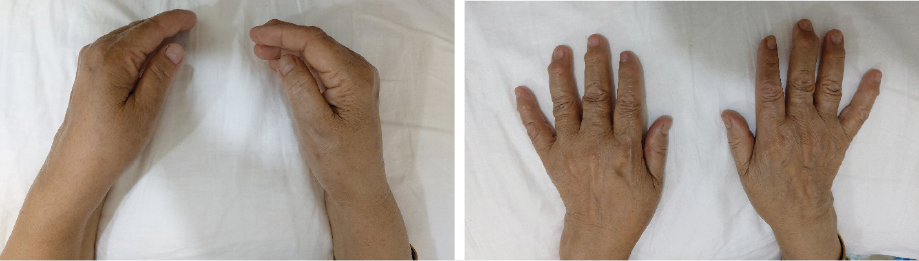 Figure 1: Hands and wrist joints show swelling over the medial carpometacarpal joints and Heberden and Bouchard nodules.
Figure 1: Hands and wrist joints show swelling over the medial carpometacarpal joints and Heberden and Bouchard nodules.
Laboratory investigations showed normal total leukocyte count, urinalysis, creatinine, transaminase, calcium, phosphorus, and uric acid levels. Rheumatoid factor was negative, and C-reactive protein level was mildly raised (10 mg/L; ref < 5). Joint fluid aspirated from the knee and wrist was non-inflammatory. Radiological images post-flare-up revealed signs of osteoarthritis in the proximal and distal interphalangeal joints of the hands, the trapeziometacarpal joint, and both medial tibiofemoral joints [Figure 2].
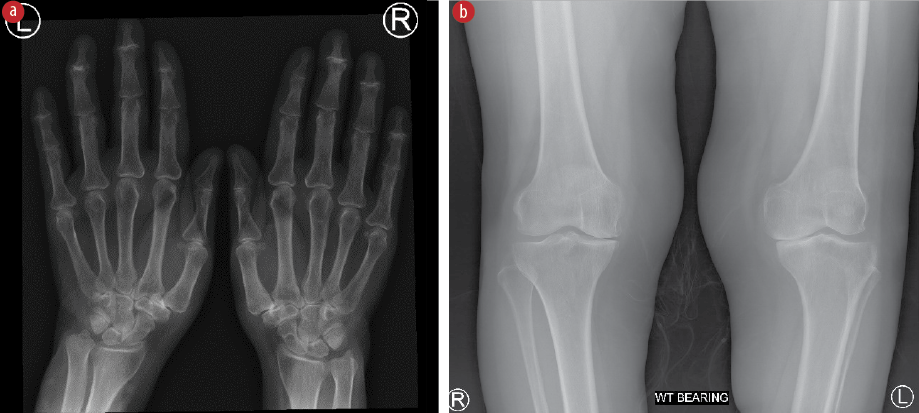 Figure 2: (A) X-ray image of hands and wrists showing reduced joint space with degenerative changes in proximal and distal interphalangeal joints of the hands, and trapeziometacarpal joints. (B) X-ray of the knees showing reduced joint spaces and degenerative changes in the medial tibiofemoral joints.
Figure 2: (A) X-ray image of hands and wrists showing reduced joint space with degenerative changes in proximal and distal interphalangeal joints of the hands, and trapeziometacarpal joints. (B) X-ray of the knees showing reduced joint spaces and degenerative changes in the medial tibiofemoral joints.
A diagnosis of flare-up of osteoarthritis secondary to zoledronic acid infusion was made. The patient was treated symptomatically with paracetamol and codeine. Her symptoms gradually resolved, taking four to five weeks for a full recovery. Informed consent was obtained from the patient.
Discussion
The first documented evidence of the effectiveness of zoledronic acid was provided by the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial, a placebo-controlled randomized controlled study.4 Another large multi-center trial comparing zoledronic acid IV infusion with oral alendronate documented evidence favoring the former.5 Long-term studies have also reported the effectiveness and favorable safety profile of zoledronic acid when administered once yearly over five years.6 The Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Fracture Recurrent Trial reported that the annual infusion of zoledronic acid was significantly associated with reduced risk of new fractures and a higher survival rate.7
In a minority of patients, zoledronic acid may cause temporary adverse effects, or acute phase responses (APR), including fever with chills (16.1%), myalgia (9.5%), flu-like symptoms (7.8%), headache (7.1%), and arthralgia (6.3%).3,4,8,9 Most APRs develop within 48 hours post zoledronic acid infusion, and tend to be self-limiting and disappear within a few days.9 Rarely, APR events may be severe and life-threatening, requiring urgent hospitalization and prolonged care, especially for cancer patients and elderly individuals with multiple comorbidities.10
We identified two case reports in the literature that are comparable to ours. Both reported severe musculoskeletal adverse effects, including the onset of new arthritis and flare-up of existing osteoarthritis, requiring hospitalization and continuum of care [Table 1].11,12 In the first case, a single infusion of zoledronic acid for osteoporosis triggered a flare-up of osteoarthritis symptoms in both hands. The patient was treated with nonsteroidal anti-inflammatory drug and steroids and recovered. The second infusion of zoledronic acid caused relatively mild symptoms, which resolved quickly.11 In the second case, the initial zoledronic acid infusion caused a severe flare-up of osteoarthritis of the hand, wrist, ankle, and foot, causing significant disability and required long-term care and physiotherapy to resolve.12 However, unlike our patient, both had significant comorbidities.
Table 1: Summary of case reports of osteoarthritis flare-upsv following initial zoledronic acid infusion.
|
Werner de Castro
et al,11
|
Brazil
|
Osteoporosis,
osteoarthritis.
|
5 mg IV to treat osteoporosis.
|
Interphalangeal and trapeziometacarpal joints of both hands.
|
One day after zoledronic acid IV.
|
Three days
|
Paracetamol 3 g/day,
aceclofenac 200 mg/day, and intramuscular corticosteroids (betamethasone phosphate 2 mg and betamethasone dipropionate 5 mg)
|
IV.: intravenous.
Zoledronic acid infusion has also been reported to trigger ocular complications, renal impairment, hypocalcemia, and pseudo-gout attacks.6,8,10,13 Interestingly, even low doses of zoledronic acid are known to trigger severe reactions. Hence, it is challenging to predict the likelihood of serious adverse effects occurring in individual cases.10,11
Conclusion
We described a patient with osteoporosis (and without significant co-morbidities), who, following a single infusion of zoledronic acid, developed a flare-up of her existing osteoarthritis, causing severe pain and disability. Symptomatic treatment and supportive care are required for such rare patients.
Disclosure
The authors declare no conflicts of interest.
references
- 1. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical pracice. Mayo Clin Proc 2008 Sep;83(9):1032-1045.
- 2. He B, Zhao JQ, Zhang MZ, Quan ZX. Zoledronic acid and fracture risk: a meta-analysis of 12 randomized controlled trials. Eur Rev Med Pharmacol Sci 2021 Feb;25(3):1564-1573.
- 3. Dhillon S. Zoledronic acid (Reclast®, Aclasta®): a review in osteoporosis. Drugs 2016 Nov;76(17):1683-1697.
- 4. Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of post-menopausal osteoporosis. N Engl J Med 2007;356(18):1809-1822.
- 5. McClung M, Recker R, Miller P, Fiske D, Minkoff J, Kriegman A, et al. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone 2007 Jul;41(1):122-128.
- 6. Devogelaer JP, Brown JP, Burckhardt P, Meunier PJ, Goemaere S, Lippuner K, et al. Zoledronic acid efficacy and safety over five years in postmenopausal osteoporosis. Osteoporos Int 2007 Sep;18(9):1211-1218.
- 7. Lyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007 Nov;357(18):1799-1809.
- 8. Kotian P, Boloor A, Sreenivasan S. Study of adverse effect profile of parenteral zoledronic acid in female patients with osteoporosis. J Clin Diagn Res 2016 Jan;10(1):OC04–OC06.
- 9. Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab 2010 Sep;95(9):4380-4387.
- 10. Powell D, Bowler C, Roberts T, Garton M, Matthews C, McCall I, et al. Incidence of serious side effects with intravenous bisphosphonate: a clinical audit. QJM 2012 Oct;105(10):965-971.
- 11. Werner de Castro GR, Neves FS, de Magalhães Souza Fialho SC, Pereira IA, Ribeiro G, Zimmermann AF. Flare-up of hand osteoarthritis caused by zoledronic acid infusion. Osteoporos Int 2010 Sep;21(9):1617-1619.
- 12. White SL, Jacob A, Gregson C, Bhalla A. Severe polyarthritis secondary to zolendronic acid: a case report and literature review. Clin Cases Miner Bone Metab 2015;12(1):69-74.
- 13. Hill AC, Al Asmar R, Olajide AA, BenHamed N. A case of pseudogout following zoledronic acid administration. Cureus 2021;13(6):e15627.