Immunoglobulin A deficiency (IgAD) is the most inborn error of immunity worldwide.1 It presents as selective (SIgAD) in patients who have low IgA with either (a) normal immunoglobulin M (IgM) and immunoglobulin G (IgG) levels, or (b) other immunoglobulin deficiencies.1 SIgAD is defined as an isolated serum IgAD in the presence of normal IgM and IgG.1
The most significant risk factor for IgAD is a family history of IgAD or common variable immunodeficiency. First-degree relatives may have an approximately 50-fold increased risk, with maternal transmission being more common.1 Most patients with SIgAD remain asymptomatic; less than one-third present with recurrent infections, such as sinopulmonary and gastrointestinal infections, commonly associated with giardiasis, autoimmune conditions, allergic disorders, or anaphylactic transfusion-related reactions.1,2
The global prevalence of SIgAD, one of the most common inborn immunity disorders, varies significantly by ethnicity.1 Among Caucasians, the overall prevalence is approximately one in 500 individuals (0.2%);3 studies reported a prevalence of one in 163 (0.6%) in Spain;4 one in 252 (0.4%)in Nigeria;5 one in 875 (0.1%) in England;6 and one in 965 (0.1%) in Brazil.7 A 2002 study in the US reported that 2–3% of celiac disease patients had IgAD, representing a 10–15-fold higher prevalence than in the general population.8 Interestingly, the prevalence is significantly lower among East Asian populations, with one in 1615–5000 (0.06–0.02%) in China9,10 and one in 14 840 in Japan (0.01%).11
A significantly high prevalence of SIgAD, one in 143 (0.7%), has been reported from the Arabian Peninsula, which is partly attributed to ethnicity and a higher rate of consanguinity.12 Saudi Arabia has an estimated prevalence of IgAD of 7/1000 (0.7%).12 In Oman, Al-Tamemi et al,13 found that one in 90 (1.1%) patients with primary immunodeficiency had SIgAD. Al Farsi et al,14 reported an SIgAD prevalence of two in 239 (0.8%) among Omani patients with immunodeficiency. Despite such high rates, apart from these two studies, there has been no other specific investigation on the prevalence of IgAD in the Omani population.
Given the above factors, along with an observed increase in referrals related to low IgA levels detected incidentally on celiac disease screening, we amied to investigate the prevalence of IgAD and SIgAD in Omani patients undergoing evaluation for celiac disease and assess adherence to follow-up testing for confirmation. Our findings will support patients counseling on management of this condition, and guide resource allocation—for example, whether to add a reflex test to screen individuals with IgAD.
Methods
A cross-sectional cohort study was conducted at the Royal Hospital, a tertiary hospital in Muscat, Oman, from January 2005 to December 2023. The study population included Omani nationals older than one year of age who were screened for celiac disease using anti-tissue transglutaminase (anti-tTG) IgA and total IgA levels. Exclusion criteria included all patients younger than one year old due to the possibility of transient hypogammaglobulinemia in infancy, patients already diagnosed with hypogammaglobulinemia (low IgA, IgG, and IgM), and non-Omani patients.
Demographic, clinical, and laboratory data were extracted from the hospital’s electronic medical records. These included age, sex, comorbidities (such as celiac disease, inflammatory bowel disease, irritable bowel syndrome, diabetes mellitus (DM), thyroid diseases, and chronic anemia), anti-tTG IgA, and IgA levels. All patients known to have pan-hypogammaglobulinemia, defined as a reduction in two or more immunoglobulin levels, IgA, IgG, and IgM, were excluded from the study.
Anti-tTG IgA level was measured using enzyme-linked immunosorbent assay (Euroimmun, Germany) with a cut-off determined as 20 U/mL. The quantification of IgA levels was performed at the Royal Hospital’s biochemistry laboratory using the polyethylene glycol-enhanced immunoturbidimetric method. The analytical system employed for this purpose utilized the Abbott assay (Abbott, USA) until 2019 and the Siemens Atellica assay (Siemens, Germany) from 2020. The polyethylene glycol -enhanced immunoturbidimetric method provides a sensitive and accurate measurement of IgA levels, ensuring reliability and consistency in analysis. The age-based cutoff values were determined according to the CALIPER Pediatric Reference Intervals (7th edition),15 and are described in Table 1.
Table 1: Immunoglobulin A in reference intervals (for both sexes) for different age groups.
|
1–2
|
0.33–1.7
|
|
2–6
|
0.37–1.78
|
|
6–14
|
0.58–2.51
|
Data were analyzed using IBM SPSS Statistics (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.), employing descriptive and, where appropriate, inferential statistical methods. Statistical significance was set at p < 0.05.
The study was approved by the Royal Hospital Ethical Committee (MoH/CSR/23/27616) and conducted in accordance with the Declaration of Helsinki.
Results
Data of 12 845 patients who underwent screening for celiac disease were retrieved from the hospital’s electronic medical records. After excluding 3230 duplicates, the remaining 9615 cases were further screened for IgA level < 0.7 g/L, regardless of age, resulting in the exclusion of 9089 patients. The remaining 526 patients were further screened for low IgA based on age reference, leading to the exclusion of an additional 399 patients. Of the remaining 127 patients with low IgA, 13 known cases of hypogammaglobulinemia were excluded. Figure 1 shows the selection process.
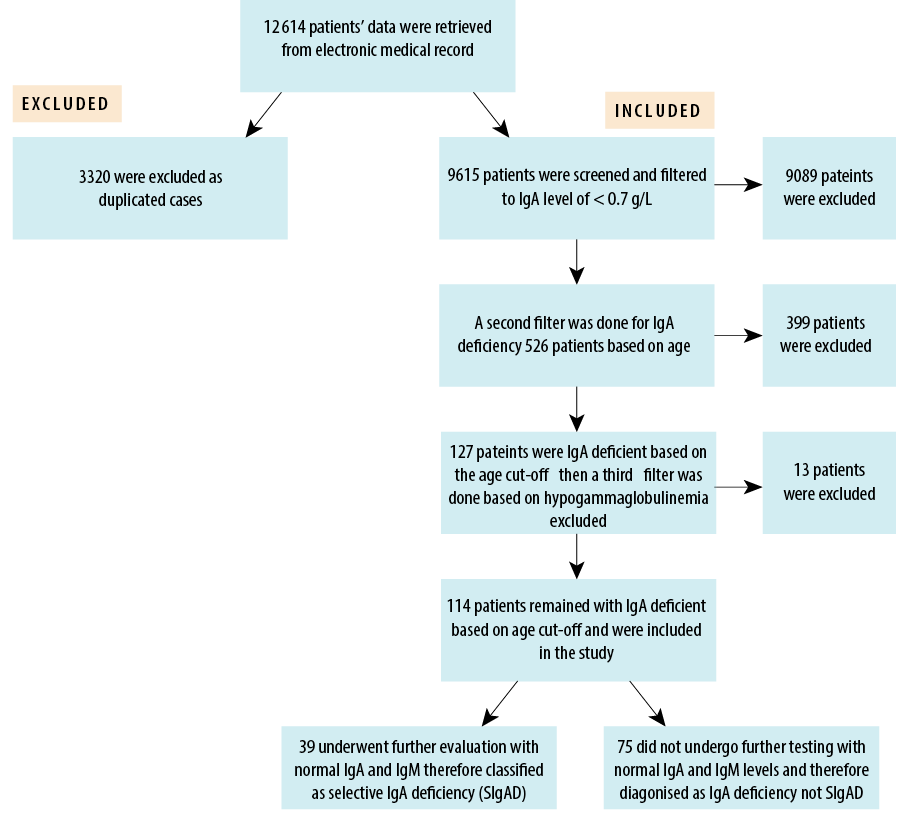 Figure 1: Participant selection process (N = 114).
Figure 1: Participant selection process (N = 114).
Accordingly, the study participants comprised 114 (1.2%) patients with low age-appropriate IgA levels. Among them, only 39 (34.2%) patients—0.4% of all screened patients—had confirmed SIgAD (normal IgG and IgM levels) by undergoing follow-up tests. Thus, the majority (n = 75; 65.8%) of patients selected for the study lacked follow-up IgG and IgM test data.
Table 2 presents the demographic characteristics of the participants. Most patients with IgAD were > 14 years old (n = 59; 51.8%), while 15 (13.2%) were aged 1–2 years, 17 (14.9%) were 2–6 years old, and 23 (20.2%) were 6–14 years old. There was no significant sex disparity in any age group.
Table 2: Demographic characteristics of Omani patients with immunoglobulin A deficiency (N = 114).
|
1–2
|
5 (4.4)
|
10 (8.8)
|
15 (13.2)
|
0.500
|
|
2–6
|
10 (8.8)
|
7 (6.1)
|
17 (14.9)
|
0.310
|
|
6–14
|
10 (8.8)
|
13 (11.4)
|
23 (20.2)
|
1.000
|
|
> 14
|
26 (22.8)
|
33 (28.9)
|
59 (51.8)
|
1.000
|
Only 39 (34.2%) participants with negative anti-tTG IgA and low IgA levels underwent IgG and IgM testing to confirm selective IgAD. Among these, only 11 (28.2%) underwent the recommended anti-tTG IgG test, with only one positive case confirmed as celiac disease by biopsy. Of the remaining 75 patients with low IgA and negative anti-tTG IgA, none underwent further IgG and IgM testing or anti-tTG IgG. Thirteen (17.3%) patients proceeded directly to esophagogastroduodenoscopy (EGD) with four (5.3%) confirmed to have celiac disease on histopathology [Table 2]. Patients exhibiting anti-tTG IgG and undergoing EGD had a significantly higher propensity for low IgA levels
(p < 0.05) [Table 3].
Table 3: Celiac disease work-up in patients with low immunoglobulin A deficiency (IgAD) (N = 114).
|
Confirmed SIgAD
|
39 (100)
|
11 (28.2)
|
1 (2.6)
|
1 (2.6)
|
28 (71.8)
|
|
Low IgA, IgG/IgM not checked
|
0 (0)
|
0 (0)
|
13 (17.3)
|
4 (5.33)
|
62 (82.7)
|
|
Total low-IgA patients
|
114 (100)
|
11 (9.6)
|
14 (12.3)
|
5 (4.4)
|
90 (78.9)
|
IgG: immunoglobulin G; IgM: immunoglobulin M; SIgAD: selective IgA deficiency; anti-tTG IgG: anti-tissue-transglutaminase antibody;
EGD: esophagogastroduodenoscopy.
Table 4: Common comorbidities in male and female patients with immunoglobulin A deficiency (N = 114).
|
Type I DM
|
14 (12.3)
|
10 (8.8)
|
24 (21.1)
|
0.300
|
|
Thyroid disease
|
2 (1.8)
|
6 (5.3)
|
8 (7.0)
|
0.200
|
|
Type II DM
|
3 (2.6)
|
5 (4.4)
|
8 (7.0)
|
0.700
|
|
Chronic anemia
|
3 (2.6)
|
1 (0.9)
|
4 (3.5)
|
0.600
|
|
IBD
|
2 (1.8)
|
1 (0.9)
|
3 (2.6)
|
1.000
|
DM: diabetes mellitus; IBD: inflammatory bowel disease; IBS: irritable bowel syndrome.
Of the 114 patients with IgAD, 24 (21.1%) had underlying type I DM, eight (7.0%) had type II DM, eight (7.0%) had thyroid disease, four (3.8%) had chronic anemia, three (2.6%) had inflammatory bowel disease, and three (2.6%) had irritable bowel syndrome [Table 3].
Among the 39 patients who underwent celiac screening, 24 (21.1%) had gastrointestinal symptoms. These included diarrhea in 15 (13.2%) patients, abdominal pain without bloating in four (3.5%), abdominal pain with bloating in two (1.8%), vomiting and diarrhea in one (0.9%), abdominal pain with vomiting in one (0.9%), and constipation in one (0.9%), while 21 (18.4%) patients had celiac screening requested as part of anemia workup. Thirty (26.3%) patients were screened due to underlying comorbidities, primarily diabetes and thyroid disease, but there was no clear indication for their screening for celiac disease.
Discussion
IgAD is associated with various autoimmune and inflammatory disorders of the gastrointestinal tract, with SIgAD associated with a 10–15-fold increased risk of celiac disease.16 This association may be genetic through shared human leukocyte antigen haplotypes.16 Our study revealed a 1.2% prevalence of IgAD, and among these, 0.4% were confirmed SIgAD. Patients > 6 years were significantly more likely to have IgAD.
The relatively highe prevalence of IgAD obeserved in our study may be partly attributed to the large original sample size of 9615 patients screened for celiac disease for various indications, including baseline screening for comorbidities. Another likely contributing factor is the high consanguinity in the Arabian countries, including Oman.12 Additionally, the hospital-based nature of our sample and not representative of the general population may have influenced the findings.
Studies have demonstrated that SIgAD is the most common primary immunodeficiency disorder, as indicated by community and blood donor studies.2 As a rule, if IgA levels are low and anti-tTG IgA is negative—raising the risk of a false negative—the patient should undergo anti-tTG IgG or EGD with biopsy to confirm celiac disease. Testing other immunoglobulins is also recommended to rule out hypogammaglobulinemia or selective IgAD.
However, only 39 (34.2%) patients with negative anti-tTG IgA and low IgA levels underwent IgG and IgM testing to confirm SIgAD. The remaining 62 patients received no further screening for celiac disease. This is of concern as the majority of patients (n = 90; 78.9%) were not further screened for celiac disease. Among the 24 patients who were properly investigated, five (20.8%) were diagnosed with celiac disease. This suggests that up to 18 cases may have been missed. The provisional incidence of 20.8% of celiac disease, though among a small number of patients, is significantly higher than the 2–3% reported elsewhere.2
Other findings from global data provided by the Jeffrey Model Centers Network, 8437 diagnosed SIgAD patients worldwide have prevalence rates that vary across continents: 5492 patients in Europe, 1704 in North America, 1050 in Latin America, 115 in Asia, and 76 in Africa.17 These figures highlight substantial variations in SIgAD prevalence, clearly influenced by ethnic backgrounds and geographical locations.
Previous studies in Oman on primary immunodeficiency diseases reported that phagocytic disorders were the most commonly observed conditions, followed by primary antibody deficiency; however, they did not quantify the prevalence of SIgAD.13,14 Our study contributes valuable national data on the prevalence of IgAD in Oman, and highlights the notably higher rates compared to global figures. It emphasizes the necessity of understanding regional and ethnic variations in immunodeficiency, which is essential for effective healthcare planning, diagnosis, and targeted interventions for our population.
Our study also highlights the association of IgAD with comorbidities such as type I DM, thyroid disorders, and inflammatory bowel diseases in the Omani population. These findings have both scientific and clinical relevance. To improve diagnostic accuracy and patient care, we recommend creating a referral guide advising patients to screen for other immunoglobulins in patients incidentally found to have low IgA. Reflex testing for anti-tTG when low IgA is detected will help avoid false negatives for celiac disease. Additionally, including information on recurrent infections in referrals can expedite the immunological workups.
Our study has several limitations. First, the retrospective nature of the study restricted our control over data quality, completeness, and consistency. Second, the hospital-based study population, focusing on patients who underwent screening for celiac disease using the anti-tTG tests, potentially introduced a selection bias. Third, the lack of comprehensive immunoglobulin panel screening for nearly two-thirds of the 114 low-IgA patients is a significant limitation, as it raises the possibility of undiagnosed cases of panhypogammaglobulinemia, which could potentially affect the accuracy of our results. Fourth, the inclusion of patients with active infections may have skewed the prevalence estimate. Last, many patients did not have IgG and IgM measured, hence we could not specifically identify all SIgAD cases.
Conclusion
The prevalence of IgAD among Omani patients evaluated for celiac disease was 1.2%, with 0.4% confirmed as SIgAD. Furthermore, the majority of patients with IgAD and negative celiac disease did not undergo anti-TTG IgG testing or an EGD biopsy to confirm the presence of the disease. Screening probable cases of celiac disease with a reflex test upfront and reevaluating budget allocation for the same are also recommended.
Disclosure
The authors declare no conflicts of interest. No funding was received for this study.
references
- 1. Yazdani R, Azizi G, Abolhassani H, Aghamohammadi A. Selective IgA deficiency: epidemiology, pathogenesis, clinical phenotype, diagnosis, prognosis and management. Scand J Immunol 2017 Jan;85(1):3-12.
- 2. Swain S, Selmi C, Gershwin ME, Teuber SS. The clinical implications of selective IgA deficiency. J Transl Autoimmun 2019 Nov;2:100025.
- 3. Latiff AH, Kerr MA. The clinical significance of immunoglobulin A deficiency. Ann Clin Biochem 2007 Mar;44(Pt 2):131-139.
- 4. Pereira LF, Sapiña AM, Arroyo J, Viñuelas J, Bardají RM, Prieto L. Prevalence of selective IgA deficiency in Spain: more than we thought. Blood 1997 Jul;90(2):893.
- 5. Ezeoke AC. Selective IgA deficiency (SIgAD) in eastern Nigeria. Afr J Med Med Sci 1988 Mar;17(1):17-21.
- 6. Holt PD, Tandy NP, Anstee DJ. Screening of blood donors for IgA deficiency: a study of the donor population of south-west England. J Clin Pathol 1977 Nov;30(11):1007-1010.
- 7. Carneiro-Sampaio MM, Carbonare SB, Rozentraub RB, de Araújo MN, Riberiro MA, Porto MH. Frequency of selective IgA deficiency among Brazilian blood donors and healthy pregnant women. Allergol Immunopathol (Madr) 1989;17(4):213-216.
- 8. Kumar V, Jarzabek-Chorzelska M, Sulej J, Karnewska K, Farrell T, Jablonska S. Celiac disease and immunoglobulin a deficiency: how effective are the serological methods of diagnosis? Clin Diagn Lab Immunol 2002 Nov;9(6):1295-1300.
- 9. Feng ML, Zhao YL, Shen T, Huang H, Yin B, Liu RZ, et al. Prevalence of immunoglobulin A deficiency in Chinese blood donors and evaluation of anaphylactic transfusion reaction risk. Transfus Med 2011 Oct;21(5):338-343.
- 10. Feng L. [Epidemiological study of selective IgA deficiency among 6 nationalities in China]. Zhonghua Yixue Zazhi 1992;72(2):88-90, 128.
- 11. Kanoh T, Mizumoto T, Yasuda N, Koya M, Ohno Y, Uchino H, et al. Selective IgA deficiency in Japanese blood donors: frequency and statistical analysis. Vox Sang 1986;50(2):81-86.
- 12. al-Attas RA, Rahi AH. Primary antibody deficiency in Arabs: first report from eastern Saudi Arabia. J Clin Immunol 1998 Sep;18(5):368-371.
- 13. Al-Tamemi S, Elnour I, Dennison D. Primary immunodeficiency diseases in Oman: five years’ experience at sultan qaboos university hospital. World Allergy Organ J 2012 May;5(5):52-56.
- 14. Al Farsi T, Ahmed K, Alshekaili J, Al Kindi M, Cook M, Al-Hosni A, et al. Immune dysregulation in monogenic inborn errors of immunity in Oman: over a decade of experience from a single tertiary center. Front Immunol 2022 Apr;13:849694.
- 15. Soldin SJ. Pediatric reference intervals. 7th ed. AMER ASSOC CLIN CHEMISTRY; 2011.
- 16. Agarwal S, Cunningham-Rundles C. Gastrointestinal manifestations and complications of primary immunodeficiency disorders. Immunol Allergy Clin North Am 2019 Feb;39(1):81-94.
- 17. Modell V, Knaus M, Modell F, Roifman C, Orange J, Notarangelo LD. Global overview of primary immunodeficiencies: a report from Jeffrey Modell Centers worldwide focused on diagnosis, treatment, and discovery. Immunol Res 2014 Oct;60(1):132-144.