The first case of COVID-19 in Oman was confirmed on February 3, 2020; by September 16, 2022, the cumulative cases rose to 397 993, with 4628 deaths.1 During the initial phase of the pandemic, antibiotics were commonly used for the disease treatment worldwide, most often empirically.2 This was influenced by the experiences from the H1N1 influenza pandemic in 2009, which had a high prevalence of secondary bacterial infections in patients admitted to the intensive care units (ICU).3–5 At the same time, evidence for the benefits of antimicrobial use in such patients was limited.6 Over time, concerns regarding overuse of antibiotics and risk of bacterial resistance emerged. It was hypothesized that the COVID-19 pandemic may be accelerating the threat of antimicrobial resistance due to the increased use of antibiotics, increased exposure to hospital environments, and invasive procedures used in COVID-19 treatment.
A scoping review based on 118 studies conducted during the first six months of the pandemic reported that 40–50% of antibiotic prescriptions for COVID-19 patients lacked clinical indications.6
A meta-analysis based on studies till June 2021 found that against a 5.6% bacterial coinfection rate, 61.8% of patients were prescribed antibiotics.7
International guidelines advise withholding antibiotics from COVID-19 patients without clinical evidence.6 The National Institutes of Health’s antibiotic recommendations for COVID-19 patients,8 with periodic updates, were adopted as official guidelines in Oman. These recommend empiric antibiotic treatment only for suspected sepsis, septic shock, or bacterial pneumonia, with daily reevaluation. Antibiotic treatment for patients with critical or severe COVID-19 should align with established guidelines for hospitalized patients with ventilator-associated pneumonia, hospital-acquired pneumonia, or other nosocomial infections.8
Evaluating the pattern and appropriateness of antibiotic use across healthcare systems during various waves of the pandemic and the factors that influenced their prescribing will help develop future guidelines. A retrospective study at our institution, analyzing four years of data (2018–2021), found significant overuse of broad-spectrum antibiotics during the first phase of the COVID-19 pandemic, leading to a significant rise in antimicrobial resistance within a relatively short period.9 However, there is a limited number of studies specifically evaluating the pattern and prevalence of antibiotic use among COVID-19 patients,10,11 especially pertaining to the Omicron phase.12 The present study aims to identify and assess the prescribing patterns of antibiotics among hospitalized patients with COVID-19 caused by the SARS-CoV-2 Omicron variant.
Methods
This retrospective study included hospitalized patients (≥18 years) with COVID-19 (Omicron variant) admitted to the Royal Hospital (RH), Muscat, between December 2021 and February 2022. RH is a 1200-bed tertiary acute-care center in Muscat and remains a major referral facility for COVID-19 patients. Ethical approval for the study was obtained from the hospital’s Scientific Research Committee (SRC #26/2020).
Patient data was collected from the electronic patient records of RH. Participants included all hospitalized patients ≥ 18 years old, of any nationality, diagnosed with a COVID-19 Omicron variant infection as confirmed by reverse transcriptase polymerase chain reaction or rapid antigen test, and hospitalized for > 24 hours. Patients were excluded if they were < 18 years old, discharged directly from the emergency room, discharged or died within 24 hours of hospitalization, or infected by non-Omicron variants of SARS-CoV-2.
Parameters evaluated on the use of antibiotic: type, number, duration/dose, route of administration, reported or presumed indications, and the patterns of antibiotic use in both ICU and non-ICU patients.
Patient and disease characteristics: Characteristics (age, sex, presence of comorbidities such as diabetes mellitus, hypertension, asthma, etc.) of patients who used antibiotics versus non-users were assessed. Disease details including severity, status of stay in ICU, length of hospital stay, and outcomes at 14 days were documented. COVID-19 severity was classified as mild, moderate, or severe, based on WHO’s Living Guidance for Clinical Management of COVID-19.13 Mild disease: patient has symptoms of COVID-19 without evidence of viral pneumonia or hypoxia. Moderate disease: shows clinical signs of non-severe pneumonia (fever, cough, dyspnea, fast breathing) with SpO2 ≥ 90% on room air. Severe disease: shows clinical signs of pneumonia (fever, cough, dyspnea) plus one of the following: respiratory rate > 30 breaths/min, severe respiratory distress, or SpO2 < 90% on room air. For this study, we also included critical cases to the severe disease group. Outcomes at 14 days post-admission were classified into three categories: ‘mortality,’ ‘remained hospitalized,’ and ‘recovered.’
Simultaneous infections: positive cultures of blood, urine, and endotracheal aspirates were considered COVID-19-related; other positive cultures were deemed unrelated. Any infection present alongside COVID-19 was considered as a simultaneous infection and categorized as either ‘coinfection’ or ‘hospital-acquired infection/superinfection’. Clinically significant positive cultures sampled within the first 48 hours of admission were categorized as coinfection and those sampled after 48 hours as hospital-acquired infection /superinfection.11 Individual patient cases were assessed for the presence of simultaneous infections, their characteristics, and the causative organism.
Data were analyzed using descriptive statistics and chi-square tests using R software version 4.2.2 (R Core Team, Austria). Association between variables (antibiotic use, infections, and clinical characteristics) was examined. A p-value of < 0.05 was considered statistically significant.
Results
A total of 225 patients were initially included, 49 were excluded due to incomplete or missing data, duplicated patient forms, discharge before 24 hours in the hospital, or being < 18 years old. Thus, the study included 176 (78.2%) patients with COVID-19 (Omicron variant).
Demographic and other details of the participants are listed in Table 1. Most patients (123; 69.9%) had mild disease. Older adults (≥ 61 years) accounted for half of all admissions (93; 52.8%); mean age of the participants was 59.3 ± 18.6 years. The majority of patients (139; 79.0%) had at least one comorbidity, hypertension (58; 33.0%) being the most common. At 14 days, (122; 69.3%) patients had recovered and 29 (16.5%) had died.
Table 1: Patient demographics and clinical characteristics (N = 176).
|
Sex
|
|
|
Male
|
105 (59.7)
|
|
Female
|
71 (40.3)
|
|
Age group, years
|
|
|
18–30
|
13 (7.4)
|
|
31–45
|
33 (18.8)
|
|
46–60
|
37 (21.0)
|
|
61–75
|
54 (30.7)
|
|
> 75
|
39 (22.2)
|
|
Presence of comorbidities
|
|
|
Yes
|
139 (79.0)
|
|
No
|
37 (21.0)
|
|
Severity of COVID-19 infection
|
|
Mild
|
123 (69.9)
|
|
Moderate
|
13 (7.4)
|
|
Severe
|
40 (22.7)
|
|
Stayed in intensive care unit
|
|
|
Yes
|
33 (18.8)
|
|
No
|
143 (81.3)
|
|
Hospital stay, days
|
|
|
1–3
|
61 (34.7)
|
|
4–6
|
60 (34.1)
|
|
7–9
|
15 (8.5)
|
|
10–12
|
13 (7.4)
|
|
> 12
|
27 (15.3)
|
|
Outcome at 14 days
|
|
|
Mortality
|
29 (16.5)
|
|
Recovered
|
122 (69.3)
|
Table 2 describes the 33 patients admitted to the ICU, nearly half of whom (14; 42.4%) were older than 60. Most ICU patients were men (20; 60.6%). The mortality rate (33.3%) was high at 14 days.
Table 2: Demographics and clinical outcomes of patients admitted in the intensive care unit (ICU)
(n = 33).
|
Admitted to ICU
|
33 (100)
|
|
Age, years
|
|
|
18–30
|
3 (9.2)
|
|
31–45
|
7 (21.2)
|
|
46–60
|
9 (27.3)
|
|
61–75
|
8 (24.2)
|
|
> 75
|
6 (18.2)
|
|
Sex
|
|
|
Male
|
20 (60.6)
|
|
Female
|
13 (39.4)
|
|
Outcome at 14 days
|
|
|
Mortality
|
11 (33.3)
|
|
Recovered
|
6 (18.2)
|
Simultaneous infections were rare among our 176 patients, with only 11 (6.3%) cases of simultaneous infections. Bacterial infections were confirmed in seven of these patients and fungal infection (Candida spp.) in the remaining four [Table 3].
Table 3: Common organisms isolated from COVID-19 (Omicron) patients with coinfections and superinfections (n = 11).
|
Type of infection
|
|
|
Coinfection
|
5
|
|
Superinfection
|
6
|
|
Type of organisms
|
|
|
Bacterial
|
7
|
|
Fungal
|
4
|
|
Bacteria
|
|
|
Escherichia coli
|
2
|
|
Enterobacteriaceae
|
2
|
|
Pseudomonas aeruginosa
|
2
|
|
Stenotrophomonas maltophilia
|
1
|
|
Staphylococcus aureus
|
1
|
|
Fungus
|
|
|
Candida spp.
|
4
|
|
Diagnosis
|
|
|
Sepsis
|
10 (90.9)
|
|
Urinary tract infection
|
2 (18.2)
|
|
Catheter-related bloodstream infection
|
1 (9.1)
|
The most common type of infection among individuals with simultaneous infection was sepsis (five coinfections and six superinfections) [Table 3].
The majority of patients (104; 59.1%) received at least one antibiotic during their stay in the hospital, only 11 had confirmed simultaneous infection based on culture results. Frequently used antibiotics included ceftriaxone (45.2%), piperacillin-tazobactam (45.2%), and vancomycin (21.2%) [Table 4]. All antibiotics were administered intravenously except for two patients who took them orally (azithromycin and doxycycline).
Table 4: Antibiotics administered to hospitalized patients with COVID-19 (Omicron variant)
(n = 104).
|
Ceftriaxone
|
47 (45.2)
|
|
Piperacillin + tazobactam
|
47 (45.2)
|
|
Vancomycin
|
22 (21.2)
|
|
Meropenem
|
16 (15.4)
|
|
Amoxicillin + clavulanic acid
|
15 (14.4)
|
|
Amikacin
|
1 (1.0)
|
|
Azithromycin
|
1 (1.0)
|
|
Clarithromycin
|
1 (1.0)
|
|
Doxycycline
|
1(1.0)
|
Totals exceed the number of patients as some received more than one antibiotic.
Nearly half of these patients (51/104; 49.0%) were given antibiotics for 1–3 days (median = 4 days; IQR = 1–16) [Figure 1].
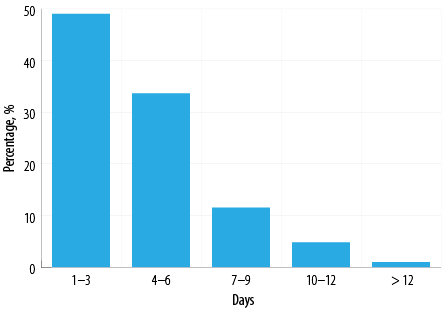 Figure 1: Durations of antibiotic treatment for patients diagnosed with COVID-19, Omicron variant (n = 104).
Figure 1: Durations of antibiotic treatment for patients diagnosed with COVID-19, Omicron variant (n = 104).
The association between simultaneous infection status and disease characteristics is shown in Table 5. Patients with simultaneous infection had a significantly higher rate of severe disease (63.6% vs. 20%; p = 0.003) and mortality at 14 days (72.7% vs. 12.7%; p < 0.001) and remained hospitalized significantly longer (≥ 10 days) (p = 0.002).
Table 5: Comparison between COVID-19 Omicron patients with versus without simultaneous infections (N = 176)
|
Total patients
|
176
|
11 (6.3)
|
165 (93.8)
|
|
|
Severity
|
|
|
|
0.003*
|
|
Mild
|
123
|
4 (36.4)
|
119 (72.1)
|
|
|
Moderate
|
13
|
0 (0.0)
|
13 (7.9)
|
|
|
Severe
|
40
|
7 (63.6)
|
33 (20.0)
|
|
|
Need for ICU
|
|
|
|
0.006*
|
|
Yes
|
33
|
6 (54.5)
|
27 (16.4)
|
|
|
No
|
143
|
5 (45.5)
|
138 (83.6)
|
|
|
Outcomes at 14 days
|
< 0.001*
|
|
Mortality
|
29
|
8 (72.7)
|
21 (12.7)
|
|
|
Recovered
|
122
|
1 (9.1)
|
121 (73.3)
|
|
|
Remained hospitalized
|
25
|
2 (18.2)
|
23 (13.9)
|
|
|
Presence of comorbidities
|
0.123
|
|
Yes
|
139
|
11 (100)
|
128 (77.6)
|
|
|
No
|
37
|
0 (0.0)
|
37 (22.4)
|
|
|
Total stay in hospital, days
|
0.002*
|
|
1–3
|
61
|
2 (18.2)
|
59 (35.8)
|
|
|
4–6
|
60
|
1 (9.1)
|
59 (35.8)
|
|
|
7–9
|
15
|
1 (9.1)
|
14 (8.5)
|
|
|
10–12
|
13
|
4 (36.4)
|
9 (5.5)
|
|
|
>12
|
27
|
3 (27.3)
|
24 (14.5)
|
|
|
Stay in ICU,† days
|
33
|
n = 6
|
n = 27
|
0.175
|
|
1–3
|
13
|
1 (16.7)
|
12 (44.4)
|
|
|
4–6
|
8
|
1 (16.7)
|
7 (25.9)
|
|
|
7–9
|
3
|
2 (33.3)
|
1 (3.7)
|
|
|
10–12
|
3
|
1 (16.7)
|
2 (7.4)
|
|
ICU: intesive care unit. *Significant; †Stay in ICU applies only to patients requiring ICU admission.
Table 6 shows the association between antibiotic use versus patient/disease characteristics. Women received significantly more antibiotics (69.0%) than men (52.4%); p = 0.030. Most patients (90.9%) with a simultaneous infection received antibiotics, compared with 94 (57.0%) patients without (p = 0.029). Notably, four of these 11 patients had a fungal infection for which antibiotics are not indicated.
Table 6: Demographics, severity, and outcomes of COVID-19 Omicron patients with or without antibiotic treatment (N = 176).
|
Total patients
|
176
|
104
|
72
|
|
|
Age group, years
|
|
|
|
0.164
|
|
18–30
|
13
|
7 (53.8)
|
6 (46.2)
|
|
|
31–45
|
33
|
17 (51.5)
|
16 (48.5)
|
|
|
46–60
|
37
|
17 (45.9)
|
20 (54.1)
|
|
|
61–75
|
54
|
36 (66.7)
|
18 (33.3)
|
|
|
> 75
|
39
|
27 (69.2)
|
12 (30.8)
|
|
|
Sex
|
|
|
|
0.030*
|
|
Male
|
105
|
55 (52.4)
|
50 (47.6)
|
|
|
Female
|
71
|
49 (69.0)
|
22 (31.0)
|
|
|
Disease severity
|
|
|
|
0.308
|
|
Mild
|
123
|
69 (56.1)
|
54 (43.9)
|
|
|
Moderate
|
13
|
10 (76.9)
|
3 (23.1)
|
|
|
Severe
|
40
|
25 (62.5)
|
15 (37.5)
|
|
|
Intensive care unit stay
|
|
|
|
0.238
|
|
Yes
|
33
|
23 (69.7)
|
10 (30.3)
|
|
|
No
|
143
|
81 (56.6)
|
62 (43.4)
|
|
|
Length of hospital stay, days
|
0.060
|
|
1–3
|
61
|
29 (47.5)
|
32 (52.5)
|
|
|
4–6
|
60
|
37 (61.7)
|
23 (38.3)
|
|
|
7–9
|
15
|
13 (86.7)
|
2 (13.3)
|
|
|
10–12
|
13
|
7 (53.8)
|
6 (46.2)
|
|
|
>12
|
27
|
18 (66.6)
|
9 (33.3)
|
|
|
14–day outcomes
|
0.196
|
|
Death
|
29
|
21 (72.4)
|
8 (27.6)
|
|
|
Recovered
|
122
|
67(54.9)
|
55 (45.1)
|
|
|
Remained hospitalized
|
25
|
16 (64.0)
|
9 (36.0)
|
|
|
Comorbidities
|
|
|
|
0.997
|
|
Yes
|
139
|
82 (59.0)
|
57 (41.0)
|
|
|
No
|
37
|
22 (59.5)
|
15 (40.5)
|
|
|
Presence of simultaneous infection
|
0.029*
|
|
Yes
|
11
|
10 (90.9)
|
1 (9.1)
|
|
*Significant.
Table 7 shows associations between the number of antibiotics used and patient/disease characteristics. Patients with severe disease were significantly more likely to receive three antibiotics (24.0%) compared to those with moderate (10.0%) or mild (1.4%) disease (p = 0.002). Significant difference was also observed between patients with severe, moderate, or mild disease who received two or more antibiotics (p = 0.002). A significantly higher percentage of ICU patients received two or more antibiotics compared to non-ICU patients (p = 0.030). The 14-day mortality rate was also significantly higher among those who received two or more antibiotics (p = 0.002).
Table 7: Number of antibiotics administered per patient compared with patient/disease characteristics
(n = 104).
|
Total patients
|
104
|
66
|
28
|
8
|
1
|
1
|
|
|
Age group, years
|
|
|
|
|
|
|
0.224
|
|
18–30
|
7
|
5 (71.4)
|
2 (28.6)
|
-
|
-
|
-
|
|
|
31–45
|
17
|
13 (76.5)
|
3 (17.6)
|
1 (5.9)
|
-
|
-
|
|
|
46–60
|
17
|
7 (41.2)
|
10 (58.8)
|
|
-
|
-
|
|
|
61–75
|
36
|
24 (66.7)
|
8 (22.2)
|
2 (5.6)
|
1 (2.8)
|
1 (2.8)
|
|
|
> 75
|
27
|
17 (63.0)
|
5 (18.5)
|
5 (18.5)
|
-
|
-
|
|
|
Sex
|
|
|
|
|
|
|
0.375
|
|
Male
|
55
|
33 (60.0)
|
16 (29.1)
|
6 (10.9)
|
-
|
-
|
|
|
Female
|
49
|
33 (67.3)
|
12 (24.5)
|
2 (4.1)
|
1 (2.0)
|
1 (2.0)
|
|
|
Disease severity
|
|
|
|
|
|
|
0.002*
|
|
Mild
|
69
|
49 (71.0)
|
19 (27.5)
|
1 (1.4)
|
-
|
-
|
|
|
Moderate
|
10
|
8 (80.0)
|
1 (10.0)
|
1 (10.0)
|
-
|
-
|
|
|
Severe
|
25
|
9 (36.0)
|
8 (32.0)
|
6 (24.0)
|
1 (4.0)
|
1 (4.0)
|
|
|
Intensive care unit admission
|
|
|
|
|
|
0.030*
|
|
Yes
|
23
|
10 (43.5)
|
8 (34.8)
|
3 (13.0)
|
1 (4.3)
|
1 (4.3)
|
|
|
No
|
81
|
56 (69.1)
|
20 (24.7)
|
5 (6.2)
|
-
|
-
|
|
|
Total stay in hospital, days
|
|
|
|
|
|
|
0.101
|
|
1–3
|
29
|
18 (62.1)
|
9 (31.0)
|
2 (6.9)
|
-
|
-
|
|
|
4–6
|
37
|
28 (75.7)
|
8 (21.6)
|
1 (2.7)
|
-
|
-
|
|
|
7–9
|
13
|
9 (69.2)
|
2 (15.3)
|
2 (15.3)
|
-
|
-
|
|
|
10–12
|
7
|
2 (28.6)
|
5 (71.4)
|
-
|
-
|
-
|
|
|
>12
|
18
|
9 (50.0)
|
4 (22.2)
|
3 (16.7)
|
1 (5.6)
|
1 (5.6)
|
|
|
Outcome at 14 days
|
|
|
|
|
|
|
0.002*
|
|
Mortality
|
21
|
8 (38.1)
|
8 (38.1)
|
4 (19.0)
|
1 (4.8)
|
|
|
|
Recovered
|
67
|
50 (74.6)
|
16 (23.9)
|
1 (1.5)
|
-
|
-
|
|
|
Remained hospitalized
|
16
|
8 (50.0)
|
4 (25.0)
|
3 (18.8)
|
-
|
1 (6.3)
|
|
|
Comorbidities
|
|
|
|
|
|
|
0.386
|
|
Present
|
82
|
53 (64.6)
|
22 (26.8)
|
6 (7.3)
|
1 (1.2)
|
-
|
|
|
Absent
|
22
|
13 (59.1)
|
6 (27.3)
|
2 (9.1)
|
-
|
1 (4.5)
|
|
|
Simultaneous infections
|
|
|
|
|
|
|
0.002*
|
|
Present
|
10
|
2 (20.0)
|
5 (50.0)
|
2 (20.0)
|
1 (10.0)
|
-
|
|
Dashes (-) indicate zero patients in that category .*Significant.
Discussion
The main finding of this study was that most COVID-19 (Omicron) patients who received antibiotics had no confirmed bacterial coinfection, suggesting there was no clear indication for their necessity. Patient demographics and disease details also offered some significant results. For example, most of our hospitalized patients were aged 61 or older, reflecting the higher severity of the Omicron variant among the elderly, consistent with Chinese14 and Japanese15 studies. However, our overall results also aligned with the reports elsewhere of the relative mildness of the Omicron variant, as most of our hospitalized patients had mild disease (69.9%).16,17
During the pre-Omicron period (March 2020 – November 2021), all COVID-19 cases admitted to RH were moderate to severe. With the emergence of the Omicron variant (December 2021 – February 2022), most cases were mild. During this period, many patients admitted for other reasons were found to be Omicron positive as well—with mild symptoms. A Japanese study reported that during the Omicron period, coinfection or deterioration of an underlying disease had a more significant effect on hospitalization than COVID-19 infection itself.15 In the present study, 69.3% of the patients recovered from the disease while 16.5% died by 14 days, somewhat high for Omicron, but explained by older age and comorbidities. In an Omicron-period study in Iran, the death rate among hospitalized patients with Omicron BA.5 variant was 6.25%.18 In California, USA, adults hospitalized during Omicron period had lower likelihood of ICU admission, perhaps due to higher proportion of vaccinated patients.19
In our cohort, only 6.3% of patients had simultaneous infections (bacterial and fungal) and only 4.0% had bacterial infection. Comparable rates have been reported in Germany (7.1%),20 though higher rates were reported elsewhere (USA: 19%; Iran:14.4%),21 but significantly lower in Pakistan (1.4%).22 A Japanese study reported significantly higher rates of coinfections in the Omicron period than in the pre-Omicron period (44.4% vs. 0.8%).15 A 2023 WHO study which included 592 898 patients from 65 countries between January 2020 and March 2023 (covering all major variants of SARS-CoV2) estimated an overall co-infection rate of 7.9%.23
In the present study, E. coli was the most prevalent pathogen (2; 18.2%), which could be related to urinary source being the second most common infection after septic shock. Data from Europe (17.5%) and USA (26%) also reflect the high prevalence of E. coli.24,25 Candida spps. was isolated in four of our patients, suggesting superinfections rather than coinfections.
Overall, 59.1% of this cohort received antibiotics similar to the Hong Kong study.12 In 2023, the WHO global review on antibiotic use showed wide inter-regional variations ranging from 83.0% in the Eastern Mediterranean Region to 32.8% in the Western Pacific Region.23 High rate of antibiotic use was also reported before the Omicron wave—USA (67%), Jordan (69%), Ireland (78.4%), and Pakistan (93.7%).22,25–27
Despite a low rate of confirmed coinfections, 59.1% of our patients received antibiotics, likely due to the challenge of ruling out bacterial infections in severely ill presentations. However, our antimicrobial stewardship team reviewed such empirical antibiotic use, which were typically discontinued within 72 hours if cultures were negative. A pre-Omicron period study in Ireland reported 66.4% of empirical antibiotic prescription for suspected pneumonia in their COVID-19 patients.27 Another early study in Pakistan (April 2021) revealed an extremely high rate of 96.3% .22
Most empiric antibiotics administered to our cohort were systemic—ceftriaxone, piperacillin and tazobactam (45.2%); vancomycin (21.2%); and meropenem (15.4%). Ceftriaxone was also the most prescribed empiric antibiotic in USA and Bangladesh.25,27 Azithromycin was the least used antibiotic in our cohort, but an Omani study by Khamis et al,10 reported it to be the preferred antibiotic, administered to 71% of COVID-19 patients early in the pandemic, till April 2020. In the early days of the pandemic, azithromycin was commonly prescribed until later studies disproved its presumed benefits. In USA, empirical use of vancomycin was common, as in our cohort.25 Early
in the pandemic (till May 2020) an Irish study by O’Kelly et al,26 found the most common empirical antibiotic prescribed to be piperacillin and tazobactam, ceftriaxone and co-amoxiclav. In another early study conducted in Jordan (October–December 2020), fluoroquinolone (31.9%), macrolide (25.0%), and third generation cephalosporin (17.6%) were prominent.28
These variations in antibiotic use across studies could be due to differences in study timing, national guidelines, drug availability, and local resistance patterns. In our study, nearly half of the patients received antibiotics for one to three days only. In the Jordanian study above, only 17.5% of patients were on antibiotics for less than three days.28 Early discontinuation because of negative microbial culture may help reduce the risk of antibiotic resistance, emphasizing the value of antibiotic stewardship.
We found that the presence of simultaneous infection was positively associated with severity of the disease, mortality, and duration of hospital stay. Similar findings were reported in a Chinese cohort comprising severe and critical hospitalized Omicron patients.14
We also noted that the antibiotic usage rate differed significantly with sex and presence of simultaneous infection. Antibiotics were more frequently used among female patients than male patients. Most of our patients with a simultaneous infection (90.9%) received antibiotics compared with 57.0% of those without. It is not clear why our female patients were more likely to receive antibiotics compared to men, which requires further investigation. Unlike our study, where no significant correlation between antibiotic use and 14-day outcome, data from Jordan found that non-survivors were more likely to be prescribed antibiotics than survivors (93.1% vs. 65.2%).28
Furthermore, we found a significant association between the number of antibiotics used and disease severity, simultaneous infection, ICU admission, and 14-day outcome. Multiple antibiotic use was positively associated with disease severity. Patients with more severe disease and/or comorbidities tend to be prescribed multiple antibiotics and treated longer. Furthermore, the prolonged hospital stay increases the risk of nosocomial infections, calling for more antibiotic use. In the scoping review by Cong et al,29 the antibiotic prescribing rate for severe COVID-19 cases was 75.3% and 48.3% compared with 75.1% and 15.5% for mild and moderate cases according to results from two study periods in the pre-Omicron phase.
Despite no significant difference in overall mortality between patients who did or did not receive antibiotics, antibiotic usage (two or more antibiotics) was more common among patients with severe disease and where death was the 14-day outcome. This may be due to simultaneous infection and comorbidities among these patients. Single antibiotic use was more likely among survivors (50.0%) than those who died (38.1%). It is possible that survivors had milder disease without comorbidities. On the other hand, in the study in Jordan, non-survivors were more likely to have been prescribed single antibiotics (55.2%) compared to survivors (34.8%).28 In the WHO’s global review, a sensitivity analysis focusing on patients without suspected or confirmed bacterial infection showed nearly twice the risk of death among mild/moderate patients receiving empiric therapy compared to those not receiving antibiotics and a 16% higher risk among severe/critical patients.2
The study has several limitations. Being single center and retrospective, our findings may not be fully generalizable. Although the RH was at the forefront in managing COVID-19 cases and formulating national guidelines, antibiotic usage patterns may vary in different regions of Oman. Additionally, our analysis of antibiotic use is limited to patients infected with the Omicron variant of the disease, for which published studies are fewer, limiting robust comparisons.
Conclusion
The key finding of this study was that in vast majority of the patients who received antibiotics, there was no reported bacterial coinfection, indicating potentially unwarranted use. Although antibiotics did not clearly affect overall outcomes, the use of two or more antibiotics was more common in severe cases and those with 14-day mortality. These findings highlight the need for stricter antimicrobial stewardship, particularly in the absence of confirmed bacterial infections. Detailed evaluation, education, and development of policies are crucial to limit unwarranted antibiotic use in the future.
Disclosure
The authors declare no conflicts of interest. No funding was received for this study.
references
- 1. World Health Organization. Oman: WHO coronavirus disease (COVID-19) dashboard with vaccination data. 2022 [cited 2022 September 19]. Available from: https://covid19.who.int/region/emro/country/om.
- 2. Yin X, Xu X, Li H, Jiang N, Wang J, Lu Z, et al. Evaluation of early antibiotic use in patients with non-severe COVID-19 without bacterial infection. Int J Antimicrob Agents 2022 Jan;59(1):106462.
- 3. Abelenda-Alonso G, Padullés A, Rombauts A, Gudiol C, Pujol M, Alvarez-Pouso C, et al. Antibiotic prescription during the COVID-19 pandemic: a biphasic pattern. Infect Control Hosp Epidemiol 2020 Nov;41(11):1371-1372.
- 4. Viasus D, Paño-Pardo JR, Pachón J, Campins A, López-Medrano F, Villoslada A, et al; Novel Influenza A (H1N1) Study Group of the Spanish Network for Research in Infectious Diseases (REIPI). Factors associated with severe disease in hospitalized adults with pandemic (H1N1) 2009 in Spain. Clin Microbiol Infect 2011 May;17(5):738-746.
- 5. Cillóniz C, Ewig S, Menéndez R, Ferrer M, Polverino E, Reyes S, et al. Bacterial co-infection with H1N1 infection in patients admitted with community acquired pneumonia. J Infect 2012 Sep;65(3):223-230.
- 6. Cong W, Poudel AN, Alhusein N, Wang H, Yao G, Lambert H. Antimicrobial use in COVID-19 patients in the first phase of the SARS-CoV-2 pandemic: a scoping review. Antibiotics (Basel) 2021 Jun;10(6):745.
- 7. Alshaikh FS, Godman B, Sindi ON, Seaton RA, Kurdi A. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: a systematic review and meta-analysis. PLoS One 2022 Aug;17(8):e0272375.
- 8. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. [cited 2022 September 21]. Available from: https://www.covid19treatmentguidelines.nih.gov/.
- 9. Pandak N, Al Sidairi H, Al-Zakwani I, Al Balushi Z, Chhetri S, Ba’Omar M, et al. The outcome of antibiotic overuse before and during the COVID-19 pandemic in a tertiary care hospital in Oman. Antibiotics (Basel) 2023 Nov;12(12):1665.
- 10. Khamis F, Al-Zakwani I, Al Naamani H, Al Lawati S, Pandak N, Omar MB, et al. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: an experience from Oman. J Infect Public Health 2020 Jul;13(7):906-913.
- 11. Pandak N, Khamis F, Al Balushi Z, Chhetri S, Al Lawati A, AbouElhamd H, et al. Low rate of bacterial coinfections and antibiotic overprescribing during COVID-19 pandemic: a retrospective study from Oman. Oman Med J 2023 Jul;38(4):e525.
- 12. Blais JE, Zhang W, Lin Y, Chui CS, Cheng VC, Cowling BJ, et al. Antibiotic use in hospitalized patients with COVID-19: a population-based study in Hong Kong. Antimicrob Steward Healthc Epidemiol 2023 Nov;3(1):e205.
- 13. World Health Organization. Living guidance for clinical management of COVID-19. Living guidance 23 November 2021. [cited 2023 March 24]. Available from: https://iris.who.int/bitstream/handle/10665/349321/WHO-2019-nCoV-clinical-2021.2-eng.pdf.
- 14. Wei YY, Wang RR, Zhang DW, Chen SH, Tan YY, Zhang WT, et al. Differential characteristics of patients for hospitalized severe COVID-19 infected by the omicron variants and wild type of SARS-CoV-2 in China. J Inflamm Res 2023 Jul;16:3063-3078.
- 15. Murakami Y, Nozaki Y, Morosawa M, Toyama M, Ogashiwa H, Ueda T, et al. Difference in the impact of coinfections and secondary infections on antibiotic use in patients hospitalized with COVID-19 between the Omicron-dominant period and the pre-Omicron period. J Infect Chemother 2024 Sep;30(9):853-859.
- 16. Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet 2022 Jan;399(10323):437-446.
- 17. Kirca F, Aydoğan S, Gözalan A, Kayipmaz AE, Özdemir FAE, Tekçe YT, et al. Comparison of clinical characteristics of wild-type SARS-CoV-2 and Omicron. Rev Assoc Med Bras (1992) 2022 Nov;68(10):1476-1480.
- 18. Salehi M, Salami Khaneshan A, Farahani AS, Doomanlou M, Arabzadeh M, Sobati A, et al. Characteristics and outcomes of COVID-19 patients during the BA.5 omicron wave in Tehran, Iran: a prospective observational study. BMC Infect Dis 2023 Apr;23(1):237.
- 19. Modes ME, Directo MP, Melgar M, Johnson LR, Yang H, Chaudhary P, et al. Clinical characteristics and outcomes among adults hospitalized with laboratory-confirmed SARS-CoV-2 infection during periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant predominance - one hospital, California, July 15-September 23, 2021, and December 21, 2021-January 27, 2022. MMWR Morb Mortal Wkly Rep 2022 Feb;71(6):217-223.
- 20. Rothe K, Feihl S, Schneider J, Wallnöfer F, Wurst M, Lukas M, et al. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: a retrospective cohort study in light of antibiotic stewardship. Eur J Clin Microbiol Infect Dis 2021 Apr;40(4):859-869.
- 21. Salehi M, Khalili H, Seifi A, Davoudi H, Darazam IA, Jahangard-Rafsanjani Z, et al. Antibiotic use during the first 6 months of COVID-19 pandemic in Iran: a large-scale multi-centre study. J Clin Pharm Ther 2022 Dec;47(12):2140-2151.
- 22. Mustafa ZU, Saleem MS, Ikram MN, Salman M, Butt SA, Khan S, et al. Co-infections and antimicrobial use among hospitalized COVID-19 patients in Punjab, Pakistan: findings from a multicenter, point prevalence survey. Pathog Glob Health 2022 Oct;116(7):421-427.
- 23. World Health Organization. WHO reports widespread overuse of antibiotics in patients hospitalized with COVID-19. 2024 [cited 2025 March 13]. Available from: https://www.who.int/news/item/26-04-2024-who-reports-widespread-overuse-of-antibiotics-in-patients--hospitalized-with-covid-19.
- 24. Papst L, Luzzati R, Carević B, Tascini C, Gorišek Miksić N, Vlahović Palčevski V, et al. Antimicrobial use in hospitalised patients with COVID-19: an international multicentre point-prevalence study. Antibiotics (Basel) 2022 Jan;11(2):176.
- 25. Goncalves Mendes Neto A, Lo KB, Wattoo A, Salacup G, Pelayo J, DeJoy R III, et al. Bacterial infections and patterns of antibiotic use in patients with COVID-19. J Med Virol 2021 Mar;93(3):1489-1495.
- 26. O’Kelly B, Cronin C, Connellan D, Griffin S, Connolly SP, McGrath J, et al. Antibiotic prescribing patterns in patients hospitalized with COVID-19: lessons from the first wave. JAC Antimicrob Resist 2021 Jun;3(2):dlab085.
- 28. Molla MM, Yeasmin M, Islam MK, Sharif MM, Amin MR, Nafisa T, et al. Antibiotic prescribing patterns at COVID-19 dedicated wards in Bangladesh: findings from a single center study. Infect Prev Pract 2021 Jun;3(2):100134.
- Alnajjar MS, Al-Tabba A, Bsoul S, Aburuz S, Saeed D, Bader A. Antimicrobial prescribing and clinical outcomes in patients with COVID-19 infection: experience of a single center in an upper middle-income country. Pharm Pract (Granada) 2022;20(1):2621.
- 29. Cong W, Stuart B, AIhusein N, Liu B, Tang Y, Wang H, et al. Antibiotic use and bacterial infection in COVID-19 patients in the second phase of the SARS-CoV-2 pandemic: a scoping review. Antibiotics (Basel) 2022 Jul;11(8):991.