Screening involves the presumptive identification of unrecognized health conditions through a test, examination, or other easily and rapidly applied procedure.1 A screening test is not intended to diagnose but rather to identify disease at an early presymptomatic stage, enabling earlier intervention and management to prevent or reduce disease-related morbidity and mortality. There are two main screening strategies: universal (population-based) screening and selective (risk factor-based) screening.
In 1968, the World Health Organization introduced the Wilson-Jungner criteria, a set of 10 items for assessing the validity of a screening program.1,2 In 2003, the UK National Screening Committee issued a set of 22 criteria.2 Serum alanine transaminase (ALT), part of liver function tests, is frequently obtained in clinical practice to assess and diagnose chronic liver diseases (CLDs), such as liver cirrhosis and liver failure, which increase the risk of liver cancer. The term liver function test is misleading when applied to liver enzymes since these enzymes, including ALT, primarily assess liver damage rather than function. ALT is the most reliable, sensitive, and specific marker of liver disease compared to other liver enzymes.3–5
ALT testing is a widely accessible and cost-effective blood test that healthcare providers use to detect liver diseases. It plays a crucial role in detecting asymptomatic liver diseases, including viral hepatitis and nonalcoholic fatty liver disease (NAFLD), which represent a significant but largely undiagnosed global epidemic. Despite its importance, its use as a screening tool in primary care settings remains limited due to limited specificity, need for clinical interpretation, cost-efficiency concerns, risk of overdiagnosis, resource limitations, and the primary focus on symptomatic patients. These factors collectively hinder its widespread implementation in primary care.
A United States population survey found that the prevalence of ALT elevations (ALT > 43 IU/L) was 8.9% and 7.3% among individuals without hepatitis C virus (HCV) or excessive alcohol consumption, respectively.6 Although most individuals have benign conditions, a subgroup has significant underlying liver diseases requiring further evaluation and therapeutic intervention. Applying the 10 elements of the Wilson-Jungner criteria, we highlight the utility of routine ALT measurement as a screening tool in primary care for the early detection of significant liver disease.7
Over the past decade, significant changes in the epidemiology of liver diseases have occurred globally, with shifting prevalence in conditions such as NAFLD, alcoholic liver disease, and hepatitis C, influenced by rising obesity rates, changes in alcohol consumption patterns, and advances in antiviral therapies.8 Concurrently, advancements in diagnostic technologies, including novel biomarkers, enhanced imaging techniques, and improved non-invasive methods for assessing liver fibrosis and inflammation, have emerged. Updated guidelines from major health organizations reflect new evidence and recommendations for detecting and managing liver diseases, requiring primary care providers to align their practices with current standards.9
The role of ALT as a primary screening tool is under scrutiny. New evidence suggests alternative or adjunctive markers to improve screening efficacy, prompting primary care settings to adapt these insights to optimize protocols.10,11
Given that liver diseases significantly contribute to the global burden of disease, early detection and management in primary care are crucial to reducing the progression to severe conditions, decreasing healthcare costs, and improving quality of life. Regular updates incorporating the latest research findings ensure that primary care providers use the most current and effective strategies, directly translating into better patient outcomes, reduced incidence of advanced liver disease, and improved healthcare system efficiency. Ongoing education and training based on updated literature are essential for healthcare providers to remain informed and competent in applying new evidence to clinical practice. Thus, updating the primary care approach to screening for liver diseases by incorporating recent research findings is essential to improve patient outcomes and healthcare efficiency and align with current clinical guidelines, empowering primary care providers to provide high-quality, evidence-based care in the evolving landscape of liver disease management.
CLDs, particularly those caused by hepatitis B virus (HBV) infection, are a significant health burden in Oman.12 Over 50% of patients with liver cirrhosis in Oman showed evidence of past or present HBV infection. HBV caused cirrhosis in 26.74% of cases.12 Before the introduction of the hepatitis B vaccine in 1990, the estimated prevalence of chronic hepatitis B in Oman was 2–7%. By 2005, the vaccine coverage rate exceeded 95%. Despite high vaccination rates, serological markers of chronic hepatitis B remain common among liver cirrhosis patients in Oman. CLD and cirrhosis substantially contribute to mortality in Oman, with limited improvements despite medical advancements. The overall mortality rate was approximately 40% during the follow-up period in one study.13 Hepatic encephalopathy, mechanical ventilation, and intensive care admission were strongly associated with increased mortality in cirrhosis patients in Oman.13 An update from the global perspective is needed to lay the groundwork for prevention in Oman.
This update aims to answer the following questions: (1) what is the significance of liver disease screening in primary care settings? (2) what is the significance of ALT as a biomarker of liver disease? (3) what is the significance of ALT as a predictor of overall health outcomes? (4) what are the normal values of ALT? (5) what is the role of ALT as a screening test for liver disease? (6) when should you screen for liver diseases? (7) when is it appropriate to refer to a hepatologist? (8) what are the limitations of ALT as a screening test for CLD?
What is the significance of screening for liver diseases in primary care settings?
CLDs progress from the early stages of hepatitis to fibrosis, ultimately leading to liver cirrhosis and hepatocellular carcinoma (HCC). In 2021, coronary heart disease ranked as the ninth leading cause of death in the USA.14,15 Worldwide, HCC is the third most common cancer and the third leading cause of cancer-related death.16 In recent years, there has been a notable increase in the incidence and mortality associated with HCC in the USA. This further highlights the importance of CLD as a public health problem.17,18 One of the main reasons for the increased morbidity and mortality associated with CLD is its presentation at an advanced stage and usually with complications such as ascites, liver encephalopathy, variceal bleeding, or HCC. Most patients with CLD are asymptomatic in the early stages of their disease. Once they become symptomatic, that usually means that they have already reached a very advanced stage.
Furthermore, most CLDs are treatable and their complications can be prevented if detected early. The period between the onset of the disease (usually hepatitis) and end-stage liver disease (cirrhosis) is measured in years, if not decades, depending on the etiology of liver disease and individual patients. Therefore, detecting CLD in primary care is crucial because it can detect conditions early, allowing timely intervention and management. Early detection can mitigate disease progression, prevent complications, and improve patient outcomes.
ALT as a biomarker of liver disease
The ALT test is a simple, readily available, acceptable, and low-cost blood test. Serum ALT is the most specific and sensitive indicator of CLD.3–5 Increased serum ALT signifies hepatocellular inflammation, a pathological hallmark observed in a spectrum of liver pathologies [Table 1]. A USA study demonstrated that 88% of asymptomatic volunteer blood donors with elevated ALT do not have identifiable causes.19 In Scandinavia, a study involving 151 consecutive patients with mild to moderately increased liver transaminases without symptoms for at least six months found that identifiable causes of liver disease were more common.20 Diagnoses included NAFLD in 42%, chronic HCV in 15.3%, alcoholic liver disease in 8%, and autoimmune hepatitis in 1.3%. Serum ALT level should dictate the urgency and extent of further investigation and when to refer the patient to a specialist.5,21
Table 1: Causes of mild hypertransaminasemia (up to five times limit of normal), clinical clues, and initial diagnostic testing.3,21,22
|
Common causes
|
|
|
|
Drugs (including prescribed, over the counter, illicit drug use and herbals)
|
Lack of illness before taking the drug.
Onset of the clinical illness or biochemical abnormalities coincidental with starting culprit agent.
Improvement after the withdrawal of the drug/herb.
|
Accurate history
Roussel Uclaf Causality Assessment Method Score
Negative infective hepatitis screening, markers of autoimmune and metabolic hepatitis.
|
|
Alcohol abuse
|
Excessive alcohol consumption, AST/ALT ratio ≥ 2.0
|
Accurate history, CAGE questionnaire (cut down, annoyed, guilty, and eye-opener) for alcohol use, AST/ALT ratio, GGT.
|
|
Nonalcoholic fatty
liver disease
|
Evidence of metabolic syndrome (dyslipidemia, hypertension, diabetes, or central obesity).
AST/ALT ratio < 1.0
|
Fasting lipid profile and glucose level; consider ultrasonography to detect hepatic steatosis and fibrosis-4 test to detect fibrosis.
|
|
Hepatitis B
|
High-risk factors include Immigration from endemic countries, high-risk sexual behavior, and intravenous drug use.
|
Hepatitis B surface antigen, hepatitis B surface antibody, hepatitis B core antibody.
|
|
Hepatitis C
|
Parenteral exposure (blood transfusions, intravenous drug use, occupational), tattoos, body piercing, and high-risk sexual behavior.
|
Hepatitis C virus antibody testing.
|
|
Hereditary hemochromatosis
|
Family history
|
Transferrin saturation and ferritin level.
|
|
Less common causes
|
|
|
|
Autoimmune hepatitis
|
Personal or family history of other autoimmune diseases.
|
Immunoglobulin G levels, serum protein electrophoresis, antinuclear antibodies, smooth muscle antibodies, and liver-kidney microsomal antibodies testing.
|
|
Wilson’s disease
|
Younger than 40 years, neuropsychiatric symptoms, Kayser-Fleischer rings.
|
Serum ceruloplasmin level and ophthalmologist consultation to rule out Kayser-Fleischer rings.
|
|
α1-antitrypsin deficiency
|
Early-onset emphysema, family history.
|
Serum α1-antitrypsin level and serum protein electrophoresis.
|
|
Non-hepatic causes
|
|
|
|
Muscle disorders
|
Muscle weakness and pain, strenuous exercise.
|
Disproportionately high AST, elevated creatine kinase and aldolase levels.
|
|
Thyroid disorders
|
Signs and symptoms of hypo- or hyperthyroidism.
|
Thyroid-stimulating hormone level
|
AST: aspartate aminotransferase; ALT: alanine transaminase; GGT: gamma-glutamyl transferase
Roussel Uclaf Causality Assessment Method
The Roussel Uclaf Causality Assessment Method approach has proven to be a cost-effective and efficient way of determining the diagnosis of drug-induced liver injury. It is recommended to recheck ALT levels if they are < 5 times the upper limit of normal (ULN) before performing a detailed study. Additional evaluation is warranted if ALT remains persistently elevated for at least three to six months. Elevated ALT levels > 5 times the ULN indicate a potentially severe and active liver disease, which requires immediate evaluation without waiting for confirmation of a continued ALT abnormality. ALT levels > 10 times the ULN suggest severe acute liver cell injury, which requires a quick evaluation. The causes of severe acute liver injury (ALT levels ≥ 10 times ULN) are relatively few. Acute viral hepatitis, ischemic hepatitis, or toxin-related hepatitis should always be considered. Other possible causes are acute autoimmune hepatitis, acute biliary occlusion, or acute Budd-Chiari syndrome. The diagnosis can be made for historical reasons or the result of blood tests or abdominal imaging [Table 1].
ALT as a predictor of overall health-based outcomes
ALT also plays a crucial role beyond liver health, indicating its potential to precict general health outcomes.5 Researchers have associated elevated ALT levels with various health conditions, including cardiovascular disease, diabetes, and metabolic syndrome.23 Multiple observational studies conducted in various populations have demonstrated a robust association between elevated ALT activity and increased all-cause mortality, as well as mortality specifically from liver disease, cardiovascular disease, diabetes, and cancer.5,23,24
To create this association, a large-scale longitudinal population-based cohort study conducted in South Korea investigated > 140 000 participants aged 35–59 years for a maximum follow-up period of 10 years. This study reported a significant increase in all-cause mortality and mortality, specifically liver disease, in participants with ALT values > 20 IU/L.25 Furthermore, even within the currently accepted normal range (35–40 IU/L), higher ALT levels have been associated with an increased risk of mortality from cardiovascular diseases or all causes.5,26–29 This increase in mortality may partly stem from undetected liver disease or non-liver-related risks, such as metabolic syndrome in patients with NAFLD or those who consume alcohol. Furthermore, ALT could indicate a pro-inflammatory state that increases cardiovascular risk, especially in people with metabolic syndrome.22
In contrast, studies have found that low ALT levels correlate with increased long-term mortality, suggesting a U-shaped relationship between ALT activity and mortality.30 Low normal ALT values have been predictive of an increased risk of all-cause mortality.31 Low levels of ALT within the normal range have also been associated with all-cause mortality.30 Although the mechanisms behind the increase in mortality in individuals with significantly low levels of ALT remain elusive, a systematic review and meta-analysis have underscored this complex association, highlighting the need for more research to determine the predictive value of ALT for long-term survival.24 Therefore, both high and low levels of ALT can serve as indicators of mortality risk, but more research is necessary to understand the underlying mechanisms of these associations.
What are the normal values of ALT?
Despite the widespread use of ALT measurement in clinical practice, there is no universally accepted definition or standardization of normal reference values.5 A reliable population-based ULN for ALT is necessary for effective screening of liver diseases. Generally, the reference range for any routine laboratory test is established using values from healthy individuals and health-related results.32
In the 1980s, many laboratories established current ULN values for ALT, which served as a surrogate marker for detecting hepatitis C among blood donors before anti-HCV tests were introduced. This explains the variation in ULN values between different laboratories. “Apparently healthy” individuals had increased ALT levels due to factors such as NAFLD and HCV, excess ethanol ingestion, medication use, and herbal supplements.32
An Italian study in 2002 updated the ULN to 30 IU/L for men and 19 IU/L for women by examining 6835 healthy blood donors without risk factors for liver diseases.33 Similarly, a South Korean study with over 90 000 men followed for up to 10 years found that 30 IU/L was the optimal cut-off point for predicting liver disease in men.25 This updated ULN improved sensitivity for detecting hepatitis C among blood donors, increasing from 39.7–61.1%, with a slight decrease in specificity from 97.6–92.3%.33 When evaluating individuals with elevated ALT levels using old cut-off points, 57.6% had no definite cause of liver injury, but 85.3% had ultrasound changes consistent with liver steatosis.
The heterogeneity of ULN values used by different laboratories compromises the sensitivity of liver enzyme tests in identifying underlying liver disease. This inconsistency impedes the establishment of a universally applicable ALT cut-off value that accurately predicts an increased risk of liver-related mortality. Consequently, treatment decisions, particularly for hepatitis B, become uncertain. These limitations are critical when determining the need for treatment in patients with hepatitis B.34 Subsequent studies confirmed the validity and utility of updated healthy ALT ULN thresholds (30 IU/L for men and 19 IU/L for women). Lee et al,26 proposed an ALT ULN of 33 IU/L for men and 25 IU/L for women based on a study of 1105 young Asians with biopsy-proven normal livers. Another US study using National Health and Nutrition Examination Survey databases from 1999–2002 and 2005–2008 proposed that ALT ULN is 29 IU/L for men and 22 IU/L for women.35
Recent research suggests that lower cutoff points for ALT levels have a stronger association with adverse liver-related outcomes, including HCC, liver decompensation, and liver-related mortality.36 Consequently, recent studies in prospective cohorts without identifiable risk factors for liver disease have proposed revised healthy ULN values for ALT, ranging from 29 to 33 IU/L for men and from 19 to 25 IU/L for women.21 These updated cutoff points are increasingly accepted and incorporated into clinical guidelines and research trials.
In 2021, the same group that proposed these healthy ranges for ALT conducted another large population cross-sectional study to update and validate their accuracy in detecting liver diseases. This study responded to recent changes by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) to the reference analytical methodology, the changing epidemiology of liver diseases, and the increasing prevalence of NAFLD.37 The study demonstrated that the revised healthy ULN for ALT using the IFCC standardized method is 42 IU/L for men and 30 IU/L for women. These values are higher than the previously proposed healthy ULNs but more effective in predicting liver disease, especially in patients with metabolic syndrome, such as NAFLD. However, the IFCC standardized method has not yet been universally adopted, and several laboratories still use the old non-standardized method. These two methods can result in different ALT values, leading to misdiagnosis and mismanagement.37
Similar laboratory methods should be used to establish the “healthy” ULN of ALT for any population, and individuals with risk factors for liver diseases should be excluded. While further studies are needed to confirm the recently proposed ULN for ALT using the IFCC standardized method, the American College of Gastroenterology guidelines suggest that a proper healthy normal level of ALT ranges from 29–33 IU/L for men and 19–25 IU/L for women.21 Levels above this range should be evaluated.
The role of ALT as a screening test for liver disease
ALT shows promise as a valuable screening tool for early detection of asymptomatic liver disease. The criteria proposed by Wilson and Jungner widely recognize a framework for evaluating screening tests.7 Applying these criteria to ALT as a screening test for early detection of liver disease reveals several key considerations.5 First, CLD, a significant health problem that leads to conditions such as cirrhosis and HCC, emphasizes the importance of ALT screening.14,15 Understanding the natural progression of the condition is crucial. ALT screening can detect liver disease in its pre-cirrhotic stage. Early treatment can provide more benefits than later stages, and ALT is suitable for identifying subjects with CLD in asymptomatic phases.
The acceptability of the test is essential, with ALT being as acceptable as other established screening tests. Determining intervals for test repetition and adequate health service provision are also crucial considerations. Although formal studies have not been conducted to evaluate the risks and cost-effectiveness of ALT-based screening programs, the low cost and minimal immediate risks associated with ALT suggest favorable comparisons with the benefits of early diagnosis of CLD.5 Therefore, while ALT meets many criteria for a screening test, further research is needed to optimize its implementation, including determining the optimal screening schedule and assessing cost-effectiveness. However, ALT remains an excellent screening tool for people at risk of liver disease, which warrants a prompt clinical evaluation of abnormal results.
When should you screen for liver diseases?
ALT can detect evidence of liver disease during routine annual assessment for patients in all age groups or high-risk populations.3 The utility of ALT as a screening test in the general population, regardless of the presence of risk factors, has not been well studied. Therefore, more data are needed to examine the practical impact of ALT screening implementation and its cost-effectiveness. During the waiting period for these data, ALT can be used as an excellent screening test in selected populations with a history of risk factors for liver disease.
When is it appropriate to refer the patient to a hepatologist?
To ensure comprehensive and optimal patient care, primary care physicians (PCPs) play a crucial role in identifying situations that require specialist referral. This referral process becomes particularly vital when a particular medical condition warrants targeted treatment strategies or diagnostic modalities that extend beyond the capabilities of PCPs. Elevated liver enzymes, mainly ALT, represent one of the most common reasons for gastroenterology/hepatology outpatient referrals. The demand for specialized consultation will likely increase if a high-risk group or population-wide-related liver enzyme screening is implemented, including ALT.
Serum ALT levels guide the need for further evaluation and specialist referral in patient management, as detailed above in the section ALT as a biomarker of liver diseases. A carefully constructed diagnostic approach to identify patients with elevated ALT that benefit the most from specialist consultation can reduce the additional burden on gastroenterology or hepatology clinics from such a screening program [Figure 1]. This approach also allows PCPs to systematically assess liver enzyme abnormalities and decide when specialist referral is necessary while directly managing many aspects of care.
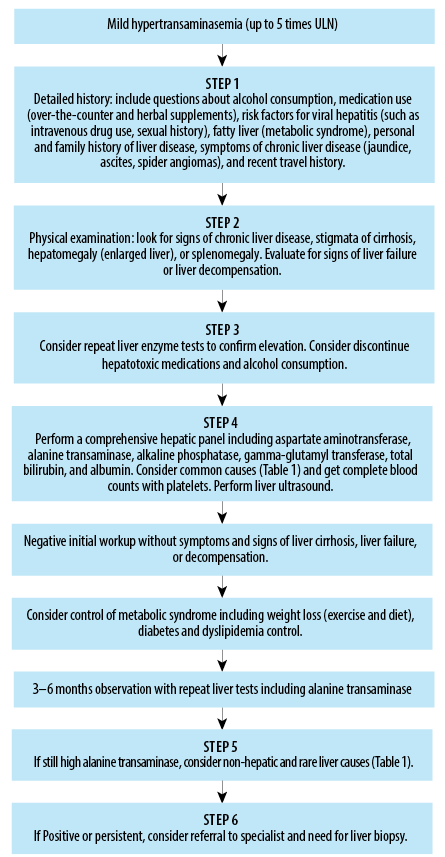 Figure 1: Management approach for mild hypertransaminasemia in primary care settings.
Figure 1: Management approach for mild hypertransaminasemia in primary care settings.
Referral to a gastroenterologist or hepatologist should be considered in cases with elevated ALT under several circumstances. The key indicators that a PCP might use to decide whether to refer a patient to a gastroenterologist or hepatologist are3:
- Patients with unexplained persistent or worsening increased ALT twice for at least three to six months despite initial treatment, including lifestyle modifications and removal of potential causative agents.
- Symptoms or signs of advanced liver disease, such as easy bleeding and swelling, itching, fatigue, jaundice, ascites, swelling of the legs, liver encephalopathy, or other indicators of cirrhosis or advanced liver disease, warrant immediate referral.
- Evidence of liver failure (hyperbilirubinemia, hypo-albuminemia, and coagulopathy).
- Evidence of liver disease that requires special care, for example, hepatitis B and C, autoimmune liver diseases, Wilson’s disease, and hemochromatosis.
- Need for advanced diagnostic or therapeutic procedures: if advanced procedures such as liver biopsy, specialized imaging beyond ultrasound, or evaluation for liver transplantation are needed.
- High-risk patients with a family history of HCC or immunocompromised syndrome may also benefit from early referral to a specialist.
Referral to a specialist facilitates specialized care where necessary and helps in a comprehensive approach to diagnosis and treatment, ensuring the best possible outcomes for patients with liver disease.
What are the limitations of ALT as a screening test for CLD?
Like other laboratory tests, ALT has limitations, including the absence of a well-accepted and uniform definition of normal ALT ranges. Therefore, it is crucial to clearly understand the distribution of ALT values in the target population and establish an agreed cut-off level.2 Recognizing that many factors beyond liver pathology can influence serum ALT levels is essential. There is a gender disparity, and men typically exhibit higher baseline ALT levels than women.33 Furthermore, research suggests that additional determinants, such as body mass index and triglyceride levels, can significantly affect ALT concentrations, regardless of sex.38,39
Men exhibit a positive correlation between total cholesterol and alcohol intake with ALT levels, while smoking, physical activity, and age demonstrate a negative correlation.39 On the contrary, in women, glucose levels are positively correlated with ALT activity, while oral contraceptive use tends to lower ALT values. In some patients with hepatitis B or hepatitis C and even some with established cirrhosis, ALT levels can fall within normal ranges. Finally, the benefits of ALT screening must outweigh the physical or psychological risks associated with false positive or false negative results, which have not yet been thoroughly studied.
Conclusion
CLDs are major contributors to morbidity and mortality globally, including in Oman. Early detection and management in primary care are critical to slowing disease progression, reducing healthcare costs, and improving patients’ quality of life. To achieve this, primary care providers must apply the most up-to-date, evidence-based strategies. ALT remains a key biomarker for evaluating liver pathology, particularly for high-risk populations such as those with NAFLD, hepatitis B, and hepatitis C. However, ALT’s limitations—especially its inability to reliably detect liver disease in the general population—underscore the need for more precise screening tools. Lower ALT thresholds and other diagnostic modalities should be considered to improve detection accuracy and outcomes. Future research should focus on validating these approaches and assessing their cost-effectiveness in broader screening programs. In the meantime, integrating ALT into a broader diagnostic framework in primary care will help identify high-risk patients earlier, reduce the burden of advanced liver disease, and optimize healthcare delivery.
Disclosure
The author declare no conflicts of interest. No funding was received for this study.
references
- 1. Wilson JM, Jungner YG. [Principles and practice of mass screening for disease]. Bol Oficina Sanit Panam 1968 Oct;65(4):281-393.
- 2. UK National Screening Committee. Criteria for appraising the viability, effectiveness and appropriateness of a screening programme. London: National Screening Committee, UK; 2003.
- 3. Minuk GY. Canadian association of gastroenterology practice guidelines: evaluation of abnormal liver enzyme tests. Can J Gastroenterol 1998 Sep;12(6):417-421.
- 4. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology 2002 Oct;123(4):1367-1384.
- 5. Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC; Public Policy Committee of the American Association for the Study of Liver Disease. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 2008 Apr;47(4):1363-1370.
- 6. Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. Am J Gastroenterol 2006 Jan;101(1):76-82.
- 7. World Health Organization. Wilson J, Jungner G. Principles and practice of screening for disease. 1968 [cited 2024 April 27]. Available from: https://apps.who.int/iris/bitstream/handle/10665/37650/WHO_PHP_34.pdf.
- 8. Xiao S, Xie W, Zhang Y, Lei L, Pan Y. Changing epidemiology of cirrhosis from 2010 to 2019: results from the global burden disease study 2019. Ann Med 2023;55(2):2252326.
- 9. Wazir H, Abid M, Essani B, Saeed H, Ahmad Khan M, Nasrullah F, et al. Diagnosis and treatment of liver disease: current trends and future directions. Cureus 2023 Dec;15(12):e49920.
- 10. Donnan PT, McLernon D, Steinke D, Ryder S, Roderick P, Sullivan FM, et al. Development of a decision support tool to facilitate primary care management of patients with abnormal liver function tests without clinically apparent liver disease [HTA03/38/02]. Abnormal liver function investigations evaluation (ALFIE). BMC Health Serv Res 2007 Apr;7:54.
- 11. Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 2000 Apr;342(17):1266-1271.
- 12. Al-Naamani K, Al-Harthi R, Al-Busafi SA, Al Zuhaibi H, Al-Sinani S, Omer H, et al. Hepatitis B related liver cirrhosis in Oman. Oman Med J 2022 May;37(3):e384.
- 13. Al Kaabi H, Al Alawi AM, Al Falahi Z, Al-Naamani Z, Al Busafi SA. Clinical characteristics, etiology, and prognostic scores in patients with acute decompensated liver cirrhosis. J Clin Med 2023 Sep;12(17):5756.
- 14. Xu J, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2021. NCHS Data Brief 2022 Dec;(456):1-8.
- 15. Kim WR, Brown RS Jr, Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology 2002 Jul;36(1):227-242.
- 16. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011 Mar-Apr;61(2):69-90.
- 17. El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med 2003 Nov;139(10):817-823.
- 18. Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, et al. Annual report to the nation on the status of cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 2016 May;122(9):1312-1337.
- 19. Kundrotas LW, Clement DJ. Serum alanine aminotransferase (ALT) elevation in asymptomatic US air force basic trainee blood donors. Dig Dis Sci 1993 Dec;38(12):2145-2150.
- 20. Mathiesen UL, Franzén LE, Frydén A, Foberg U, Bodemar G. The clinical significance of slightly to moderately increased liver transaminase values in asymptomatic patients. Scand J Gastroenterol 1999 Jan;34(1):85-91.
- 21. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol 2017 Jan;112(1):18-35.
- 22. Choi KM, Han K, Park S, Chung HS, Kim NH, Yoo HJ, et al. Implication of liver enzymes on incident cardiovascular diseases and mortality: a nationwide population-based cohort study. Sci Rep 2018 Feb;8(1):3764.
- 23. Yun KE, Shin CY, Yoon YS, Park HS. Elevated alanine aminotransferase levels predict mortality from cardiovascular disease and diabetes in Koreans. Atherosclerosis 2009 Aug;205(2):533-537.
- 24. Liu Z, Ning H, Que S, Wang L, Qin X, Peng T. Complex association between alanine aminotransferase activity and mortality in general population: a systematic review and meta-analysis of prospective studies. PLoS One 2014 Mar;9(3):e91410.
- 25. Kim HyeonChang KH, Nam ChungMo NC, Jee SunHa JS, Han KwangHyub HK, Oh DaeKyu OD, Suh Il SI. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 2004 Apr 24;328(7446):983.
- 26. Lee JK, Shim JH, Lee HC, Lee SH, Kim KM, Lim YS, et al. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology 2010 May;51(5):1577-1583.
- 27. Goessling W, Massaro JM, Vasan RS, D'Agostino Sr RB, Ellison RC, Fox CS. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology 2008 Dec;135(6):1935-1944.
- 28. Visaria A, Pai S, Fayngersh A, Kothari N. Association between alanine aminotransferase within the normal range and all-cause and cause-specific mortality: a nationwide cohort study. PLoS One 2020 Nov;15(11):e0242431.
- 29. Karaphillis E, Goldstein R, Murphy S, Qayyum R. Serum alanine aminotransferase levels and all-cause mortality. Eur J Gastroenterol Hepatol 2017 Mar;29(3):284-288.
- 30. Kawamoto R, Kikuchi A, Akase T, Ninomiya D, Tokumoto Y, Kumagi T. Association between alanine aminotransferase and all-cause mortality rate: findings from a study on Japanese community-dwelling individuals. J Clin Lab Anal 2022 May;36(5):e24445.
- 31. Ramaty E, Maor E, Peltz-Sinvani N, Brom A, Grinfeld A, Kivity S, et al. Low ALT blood levels predict long-term all-cause mortality among adults. A historical prospective cohort study. Eur J Intern Med 2014 Dec;25(10):919-921.
- 32. Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem 2000 Dec;46(12):2027-2049.
- 33. Prati D, Taioli E, Zanella A, Torre ED, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002 Jul;137(1):1-10.
- 34. Lok AS. Chronic hepatitis B. N Engl J Med 2002 May;346(22):1682-1683.
- 35. Ruhl CE, Everhart JE. Upper limits of normal for alanine aminotransferase activity in the United States population. Hepatology 2012 Feb;55(2):447-454.
- 36. Kim KN, Joo J, Sung HK, Kim CH, Kim H, Kwon YJ. Associations of serum liver enzyme levels and their changes over time with all-cause and cause-specific mortality in the general population: a large-scale national health screening cohort study. BMJ Open 2019 Jun;9(5):e026965.
- 37. Valenti L, Pelusi S, Bianco C, Ceriotti F, Berzuini A, Prat LI, et al. Definition of healthy ranges for alanine aminotransferase levels: a 2020 update. Hepatol Commun 2021 Nov;5(11):1824-1832.
- 38. Siest G, Schiele F, Galteau MM, Panek E, Steinmetz J, Fagnani F, et al. Aspartate aminotransferase and alanine aminotransferase activities in plasma: statistical distributions, individual variations, and reference values. Clin Chem 1975 Jul;21(8):1077-1087.
- 39. Piton A, Poynard T, Imbert-Bismut F, Khalil L, Delattre J, Pelissier E, et al; MULTIVIRC Group. Factors associated with serum alanine transaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. Hepatology 1998 May;27(5):1213-1219.