COVID-19 pandemic caused significant morbidity and mortality worldwide.1,2 Evidence-based therapeutic modalities for this disease are still insufficient,3 though antiviral medications have helped reduce its severity and complications.4 In addition to preventive vaccines, immunotherapies and antivirals may help individuals at moderate to high risk reduce the need for hospitalization or prevent progression to severe disease.5–7
The exact mechanisms underlying the progression of COVID-19 in the human body remain incompletely understood; however, it is known that the spike protein of the pathogen, SARS-CoV-2, binds to the angiotensin-converting enzyme 2 (ACE2) in the lower airways to facilitate entry into alveolar cells.8,9 In older adults with acute disease, dendritic cell (DC)-induced T-cell activation is delayed, and CD4+ and CD8+ T-cells exhibit a reduced capacity to produce gamma interferon (IFN-γ) and interleukin 2 (IL-2), impairing the adaptive immune response.8
Among the drugs used to mitigate the symptoms and sequelae of COVID-19 infection is umifenovir, an antiviral agent to prevent and treat a range of respiratory viral infections including influenza. Umifenovir exhibits immunomodulatory properties observed in vitro, animal models, and in human subjects. Evidence suggests that it enhances non-specific immune defenses, stimulates interferon production, and activates phagocytes. Immunological markers such as elevated blood immunoglobulin levels, increased B lymphocyte counts, and enhanced CD4+ and CD8+ cell counts improve in patients treated with umifenovir for viral respiratory tract infections characterized by reduced baseline immunity.10 Based on these findings, we conducted the present study to evaluate the efficacy and safety of umifenovir in treating COVID-19 patients.
Methods
This multicenter, controlled, open-label, parallel two-arm phase 3 randomized clinical trial included 260 patients diagnosed with COVID-19. Informed consent was received from participants before their induction into the study.
The research was conducted from April 2020 to March 2021 at two centers in Tehran, Iran—Baqiyatallah Hospital, affiliated with Baqiyatallah University of Medical Sciences (BMSU), and Sina Hospital, affiliated with Tehran University of Medical Sciences. The ethics committee of BMSU approved the clinical trial (Ref: IR.BMSU.REC.1399.037), and the study was registered with the Iranian Registry of Clinical Trials (IRCT20080901001165N46).
Inclusion criteria were as follows: (1) age > 18 years; (2) presence of at least one clinical symptom associated with COVID-19 (cough, fever > 37.5 °C, shortness of breath, weakness, myalgia, arthralgia, diarrhea, nausea, or vomiting); (3) confirmed diagnosis of COVID-19 via real-time polymerase chain reaction and/or pulmonary involvement indicated via chest computed tomography (CT) scan; (4) peripheral oxygen saturation < 93% in ambient air at rest; (5) enrollment in study within 10 days of onset of symptoms; and (6) written informed consent provided by the patient.
Exclusion criteria were (1) concurrent use of other medications with direct or potential antiviral activity against SARS-CoV-2; (2) participation in other clinical study involving investigational treatments for COVID-19; (3) pregnant or nursing women; (4) previous hypersensitivity to umifenovir; (5) respiratory failure necessitating intubation or presence of shock state/multi-organ dysfunction at baseline; (6) medical history including congenital heart disease, congestive heart failure, coronary artery disease, severe heart rhythm disorders, epilepsy, stroke, mental retardation, or spinal cord injury; and (7) immunodeficiency conditions such as organ transplants or HIV positivity.
The selected patients were randomly assigned to either the intervention or control group. The intervention group received one oral umifenovir (Arbidol™) 200 mg capsule every six hours for seven days. Both groups received standard care per national recommendations for treating novel coronaviruses at that time, including favipiravir therapy for
moderate disease.
At enrollment, demographic data, medical history, and clinical symptoms were recorded. Vital signs including blood pressure, respiratory rate (RR), heart rate, and oxygen saturation (SpO2), along with laboratory markers including complete blood count, hemoglobin, C-reactive protein (CRP), erythrocyte sedimentation rate, creatinine, and urea, were documented at baseline and on day-7. The National Early Warning Score 2 (NEWS-2), length of hospital stays, need for transfer to the intensive care unit (ICU), and hospitalization outcomes were recorded. Chest CT scans were performed at baseline and day 14 to evaluate pulmonary involvement using a semi-quantitative assessment using a visual score ranging from 0–5 assigned to each lung lobe, with a maximum possible score of 25.11
Equal numbers of participants were randomly assigned to either the umifenovir group or control group using a four-block design via www.sealedenvelope.com. This method reduced selection bias and ensured balanced representation across treatment arms.
The primary outcome was clinical improvement assessed via the NEWS-2 scoring system following the World Health Organization recommendations. NEWS-2 score identifies patients at risk of clinical deterioration through measurements including RR, SpO2, supplementary oxygen requirements, systolic blood pressure, heart rate, body temperature, and alert, verbal, pain, unresponsive scale. The alert, verbal, pain, unresponsive score was calculated based on Glasgow Coma Scale (GCS) values, where ‘A’ corresponds to GCS 14 –15; ‘V’ to GCS 9–13; ‘P’ to GCS 4–8; and ‘U’ to GCS 3. Scores from 0 to 4 indicate low risk, 5–6 medium risk, and ≥ 7 high risk.12 Secondary outcomes included changes in chest CT scan scores, duration of hospitalization, need for ICU transfer, and mortality.
Statistical analysis was conducted using SPSS Statistics (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). Normality distribution was assessed using Shapiro-Wilk test. Normally distributed continuous variables were reported as mean ± SD, while non-normally distributed continuous data was described as IQR and median. Categorical data with normal distribution was expressed as percentages. Chi-square tests and Fisher’s exact tests compared categorical data; Mann-Whitney U tests compared non-normally distributed continuous variables. Statistical significance was set at p < 0.05.
Results
Of the initial cohort of 314 patients, 121 were excluded due to non-compliance or loss of follow-up. Of the remaining 193 patients who completed the trial, 96 were assigned to the control group and 97 to the intervention group [Figure 1]. The mean age was 56.2 ± 14.9 years; males constituted 54.4% of participants. Except for age—significantly higher in the intervention group (p = 0.008) but not clinically significant—demographics and medical histories showed no statistically significant differences between the two groups at baseline [Table 1].
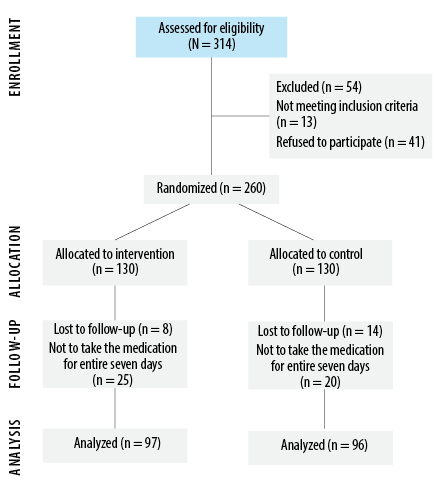 Figure 1: Flowchart of the umifenovir clinical trial.
Figure 1: Flowchart of the umifenovir clinical trial.
Table 1: Comparison of demographic information, symptoms, and characteristics between intervention group (n = 97) and control group (n = 96) in the umifenovir clinical trial.
|
Demographic information
|
|
Age, year, mean ± SD
|
59.0 ± 13.9
|
53.4 ± 15.0
|
0.080
|
|
Male sex
|
51 (53.1)
|
54 (55.7)
|
0.560
|
|
BMI, kg/m2, mean ± SD
|
27.3 ± 6.8
|
27.6 ± 4.6
|
0.691
|
|
Past medical and habitual history
|
|
Smoking
|
7 (7.3)
|
6 (6.2)
|
1.000
|
|
Diabetes mellitus
|
20 (20.8)
|
22 (22.7)
|
0.722
|
|
Hypertension
|
23 (24.0)
|
28 (28.9)
|
0.411
|
|
Chronic kidney disease
|
7 (7.3)
|
9 (9.3)
|
0.603
|
|
Ischemic heart disease
|
15 (15.6)
|
10 (10.3)
|
0.390
|
|
Malignancy
|
2 (2.1)
|
1 (1.0)
|
1.000
|
|
Asthma
|
5 (5.2)
|
1 (1.0)
|
0.211
|
|
COPD
|
2 (2.1)
|
1 (1.0)
|
1.000
|
|
Symptoms
|
|
Fever (temperature > 38 °C)
|
51 (53.1)
|
40 (41.2)
|
0.192
|
|
Chills
|
46 (47.9)
|
46 (47.4)
|
0.881
|
|
Dyspnea
|
54 (56.2)
|
53 (54.6)
|
0.883
|
|
Chest pain
|
28 (29.2)
|
24 (24.7)
|
0.740
|
|
Cough
|
55 (57.3)
|
59 (60.1)
|
0.463
|
|
Myalgia
|
43 (44.8)
|
56 (57.7)
|
0.040*
|
*Significant; BMI: body mass index; COPD: chronic obstructive pulmonary disease.
At baseline, no significant differences existed between groups regarding clinical symptoms such as fever, chills, dyspnea, chest pain, or weakness (p > 0.050), except myalgia, which was significantly higher in the intervention group [Table 2]. Both groups exhibited substantial reductions in clinical symptoms by the end of the study; however, no statistically significant differences in the magnitude of symptom reduction emerged between groups.
Table 2: Comparison of vital signs, laboratory findings, and scores at the baseline and after umifenovir clinical trial between Intervention group (n = 97) and control group (n = 96).
|
Clinical symptoms
|
|
SBP, mmHg
|
127.9 ± 16.5
|
130.4 ± 17.9
|
-4.8 ± 14.8
|
-3.7 ± 15.5
|
0.615
|
|
DBP, mmHg
|
81.4 ± 10.8
|
81.8 ± 11.8
|
-1.4 ± 9.4
|
-1.6 ± 12.2
|
0.938
|
|
HR, beats/minute
|
96.5 ± 13.4
|
94.0 ± 13.7
|
-10.2 ± 15.0
|
-8.7 ± 14.0
|
0.477
|
|
RR, beats/minute
|
17.5 ± 3.0
|
18.8 ± 3.9
|
-3.8 ± 3.7
|
-2.7 ± 2.5
|
0.010*
|
|
SpO2, %
|
92.7 ± 2. 7
|
91.5 ± 4.2
|
1.2 ± 3.4
|
2.4 ± 4.2
|
0.026*
|
|
Laboratory tests
|
|
WBC count, × 1000
|
8.8 ± 6.8
|
7.7 ± 3.7
|
-1.3 ± 4.8
|
0.7 ± 4.1
|
0.003*
|
|
Neutrophil count, × 1000
|
80.8 ± 8.7
|
77.6 ± 11.0
|
-12.0 ± 14.7
|
-8.9 ± 16.0
|
0.168
|
|
Lymphocyte count, × 1000
|
7.5 ± 4.0
|
12.8 ± 8.4
|
16.2 ± 11.4
|
10.3 ± 13.4
|
0.001*
|
|
Hb, g/dL
|
13.4 ± 2.3
|
13.8 ± 1.8
|
-1.2 ± 1.7
|
-1.0 ± 1.3
|
0.114
|
|
Platelet count, ×1000
|
212.3 ± 81.2
|
216. 6 ± 85.3
|
44.5 ± 95.1
|
46.6 ± 93.4
|
0.870
|
|
ESR, mm/hr
|
35.1 ± 23.9
|
40.7 ± 31.3
|
-17.0 ± 22.6
|
-11.5 ± 28.3
|
0.1331
|
|
CRP, mg/dL
|
35.6 ± 41.0
|
36.6 ± 43.5
|
-21.0 ± 33.7
|
-12.4 ± 24.7
|
0.045*
|
|
Cr, mg/dL
|
1.4 ± 1.2
|
1.3 ± 0.7
|
-0.2 ± 1.1
|
-0.4 ± 0.4
|
0.274
|
|
Urea, mg/dL
|
40.1 ± 33.2
|
28.5 ± 31.1
|
-11.9 ± 23.1
|
3.2 ± 20.9
|
< 0.001*
|
|
Scores changes
|
|
CT-scan score
Control, n = 68
Intervention, n = 63
|
9.7 ± 4.6
|
8.9 ± 5.9
|
-4.1 ± 3.7
|
-2.2 ± 4.4
|
< 0.001*
|
*Significant. Δ: mean2-mean1; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; RR: respiratory rate; SpO2: oxygen saturation;
WBC: white blood cell; Hb: hemoglobin; ESR: estimated sedimentation rate; CRP: C-reactive protein; Cr: creatinine; CT: computed tomography; NEWS-2: national early warning score 2.
Baseline vital signs revealed no significant differences between groups except for a higher mean respiratory rate in the intervention group and higher SpO2 in controls. However, both were clinically insignificant differences [Table 2]. Significant intergroup differences emerged post-intervention regarding white blood cell count (p = 0.003), lymphocyte count (p = 0.001), CRP (p = 0.045), RR (p = 0.010), and SpO2 (p = 0.026).
Post-intervention CT scan scores decreased significantly in both groups compared to baseline (4.1 ± 3.7 vs. 2.2 ± 4.4 for controls; 2.5 ± 3.6 vs. 2.9 ± 2.2), with comparatively more reductions in the intervention group (p < 0.001). No significant intergroup differences were noted regarding NEWS-2 score reductions, length of hospital stay, ICU admission, and mortality rate [Table 3]. None of the expected side effects of umifenovir were observed in the intervention cohort.
Table 3: Comparison of changes in secondary outcomes at the end of the umifenovir clinical trial between intervention and control groups.
|
Mortality, n (%)
|
7 (7.3)
|
8 (8.2)
|
0.873
|
|
Length of hospital stay in days,
mean ± SD
|
6.4 ± 3.6
|
6.9 ± 3.9
|
0.284
|
ICU: intensive care unit.
Discussion
This multicenter, randomized, controlled clinical trial evaluated the efficacy of umifenovir in treating COVID-19 by analyzing a total of 193 patients: 97 in the umifenovir intervention group and 96 in the control group. The baseline status was similar in the intervention and control groups, except for RR and oxygen SpO2. By the end of the study, both groups showed a significant reduction in clinical symptoms. The intervention group also demonstrated a statistically significant decrease in CT scan scores. No other significant intergroup differences were observed.
Prior to COVID-19 pandemic, umifenovir was shown to be effective against influenza in some Russian studies.10,13 However, it remains unapproved in most Western countries including the United States, where the Food and Drug Administration has not sanctioned it for influenza treatment or prevention.14
In addition to its antiviral action against influenza A and B, umifenovir is also reported to regulate the immune system by stimulating humoral immunity, interferon production, and macrophage xenophagy.13 A Russian study comparing umifenovir with oseltamivir for influenza treatment found similar mortality reduction rates for both. The advantage of umifenovir lies in its effectiveness against neuraminidase inhibitor-resistant viral strains.15 In a retrospective cohort study in China, COVID-19 patients receiving a combination of oral umifenovir and lopinavir/ritonavir showed improved chest CT scans after seven days compared to monotherapy patients.16 A systematic review by Huang et al,17 found that umifenovir significantly reduced viral load and length of hospital stay without increased side effects, but it did not lower mortality rates. In contrast, the current study did not find significant reductions in hospitalization period or mortality.
The clinical efficacy of umifenovir remains uncertain due to a lack of large-scale randomized controlled trials. Some smaller studies have produced conflicting results; for instance, Wang et al,18 suggested that umifenovir improved discharge rates and reduced mortality, whereas Huang et al,17 as well as our study, found no reduced mortality outcomes.
Additionally, some reports indicate that umifenovir is not superior to conventional supportive therapies in promoting radiological improvement or clinical recovery rates.17 Although our study noted a significant reduction in CT scan scores for patients taking umifenovir, there was no corresponding difference in clinical improvement or cure rates versus the control group. Xudan et al,19 found that umifenovir did not shorten hospitalization time in Chinese patients, which is consistent with our results.
Another study in China found that CRP, lactic acid dehydrogenase, and D-dimer levels decreased in patients who improved after umifenovir treatment but remained unchanged or increased in those who did not improve.20 This suggests these markers may correlate with disease severity and progression. In our study, white blood cell count, lymphocyte count, and CRP levels significantly decreased in the intervention group compared to the control group.
Overall, considering the current results and supportive literature, especially on those related to patient-important outcomes, there is currently insufficient justification for using umifenovir in COVID-19 patients.
This study has several limitations. First, the small sample size may limit generalizability. Second, all participating centers were within the same metropolitan area, which may not adequately represent broader demographic or geographic variations. Additionally, the research was conducted during the early phase of the pandemic (2020), when scientific understanding of COVID-19 was still evolving, potentially impacting some methodological decisions.
Conclusion
While umifenovir showed some efficacy against COVID-19 by improving certain immunological markers, our findings suggest its limited utility concerning broader patient-important outcomes. Further investigation into long-term implications is warranted for therapies targeting similar patient cohorts experiencing varying degrees of severity related to COVID-19.
Disclosure
The authors declare no conflicts of interest. Part of the study conducted at Sina Hospital was funded by the Tehran University of Medical Sciences; grant number 99-1-104-47199.
Acknowledgments
The authors extend their appreciation to the nursing staff and the participating patients for their collaboration.
references
- 1. Baloch S, Baloch MA, Zheng T, Pei X. The coronavirus disease 2019 (COVID-19) pandemic. Tohoku J Exp Med 2020 Apr;250(4):271-278.
- 2. Muralidar S, Ambi SV, Sekaran S, Krishnan UM. The emergence of COVID-19 as a global pandemic: understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochimie 2020 Dec;179:85-100.
- 3. Balkhair AA. COVID-19 pandemic: a new chapter in the history of infectious diseases. Oman Med J 2020 Apr;35(2):e123.
- 4. Wu R, Wang L, Kuo HD, Shannar A, Peter R, Chou PJ, et al. An update on current therapeutic drugs treating COVID-19. Curr Pharmacol Rep 2020;6(3):56-70.
- 5. Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, et al. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother 2020 Jun;16(6):1232-1238.
- 6. Therapeutics and COVID-19. Living guideline, 13 January 2023. [cited 2023 May 17]. Available from: https://app.magicapp.org/#/guideline/7789.
- 7. Australia H. How to treat mild COVID-19 symptoms and who can have oral antiviral treatments for COVID-19. Healthdirect Australia; 2023 [cited 2023 May 17]. Available from: https://www.healthdirect.gov.au/blog/how-to-treat-mild-covid-19-symptoms.
- 8. Harrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol 2020 Dec;41(12):1100-1115.
- 9. Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care 2020 Jul;24(1):422.
- 10. Pshenichnaya NY, Bulgakova VA, Lvov NI, Poromov AA, Selkova EP, Grekova AI, et al. Clinical efficacy of umifenovir in influenza and ARVI (study ARBITR). Ter Arkh 2019 Mar;91(3):56-63.
- 11. Francone M, Iafrate F, Masci GM, Coco S, Cilia F, Manganaro L, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol 2020 Dec;30(12):6808-6817.
- 12. Jones M. NEWSDIG: the national early warning score development and implementation group. Clin Med (Lond) 2012 Dec;12(6):501-503.
- 13. Glushkov RG, Gus’kova TA, Krylova LIu, Nikolaeva IS. Mekhanizmy immunomoduliruiushchego deĭstviia arbidola. (Mechanisms of arbidole’s immunomodulating action). Vestn Ross Akad Med Nauk 1999;(3):36-40.
- 14. U.S. Food and Drug Administration. Coronavirus (COVID-19) | Drugs [Internet]. Silver Spring (MD): FDA; 2024 Jan 31 [updated 2024 Jan 31; cited 2024 Apr 14]. Available from: https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs.
- 15. Wang Y, Fan G, Salam A, Horby P, Hayden FG, Chen C, et al. Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection. J Infect Dis 2020 Apr;221(10):1688-1698.
- 16. Deng L, Li C, Zeng Q, Liu X, Li X, Zhang H, et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J Infect 2020 Jul;81(1):e1-e5.
- 17. Huang D, Yu H, Wang T, Yang H, Yao R, Liang Z. Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Med Virol 2021 Jan;93(1):481-490.
- 18. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020 Jul;71(15):769-777.
- 19. Xudan C, Yuying Z, Baoyi Z, Jianwen Z, Wenxin H, Xi H, et al. Association of clinical characteristics and antiviral drugs with viral RNA clearance in patients with COVID-19 in Guangzhou, China: a retrospective cohort study. medRxiv 2020.04.09.20058941.
- 20. Jie X, Hongmei Y, Ping F, Kuikui Z, Bohan Y, Rui M. Beneficial effect of Arbidol in the management of COVID-19 infection. Aging (Albany NY) 2021 Apr;13(7):9253-9264.