An 11-year-old Omani boy with a known congenital factor VII deficiency presented with a four-day history of colicky abdominal pain, bilious vomiting, and constipation without gastrointestinal bleeding. When he was 14 months old, he was diagnosed with factor VII deficiency following a history of recurrent bruises and hemarthrosis with prolonged prothrombin time. The diagnosis was confirmed by identifying the presence of a homozygous pathogenic variant in the F7 gene, and since then, he has remained on on-demand factor VII replacement therapy.
There was no history of fever, abdominal trauma, bleeding from any site, jaundice, or skin pruritus. Upon examination, the patient was in pain, had a mild pallor, and was hemodynamically stable. Cardiorespiratory and chest examination were unremarkable. Abdominal examination revealed tenderness in the epigastric and umbilical regions without rebound tenderness or organomegaly. Laboratory investigations revealed low hemoglobin at 10.4 g/dL (reference range: 11.5–14.0 g/dL), platelet count of 506 × 109/L (150–450 × 109/L), and white blood cell count of 8.9 × 109/L (4.5–12 × 109/L). The prothrombin time was significantly elevated at > 180 seconds (10.3–12.1). The child had an elevated international normalized ratio of > 20 (0.9–1.2). Activated partial thromboplastin time and fibrinogen were normal. C-reactive protein level was high at 18 mg/L (< 5 mg/L). Liver enzymes, amylase, and lipase were within normal ranges.
An abdominal ultrasound revealed a hyperechoic mass measuring approximately 6.4 × 2.7 cm in the second part of the duodenum. The mass projected into the lumen of the duodenum, displacing the duodenal wall anteriorly with proximal gastric dilatation. The rest of the scan was normal [Figure 1].
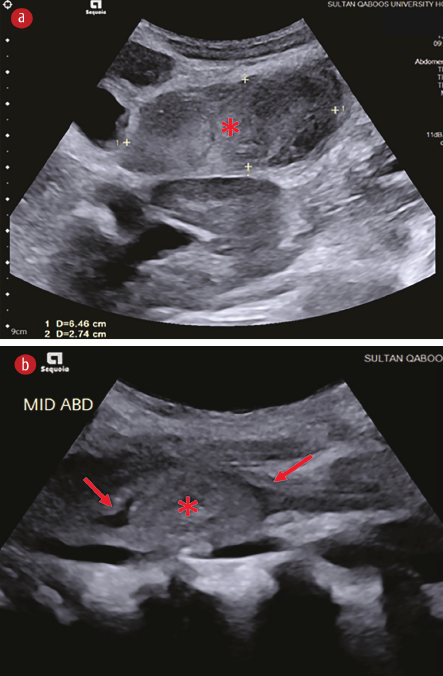 Figure 1: Ultrasound images of the upper abdomen's (a) transverse and (b) sagittal plane. Both images show a hyperechoic mass (asterisks) in the second part of the duodenum. The mass projects into the lumen of the duodenum, anteriorly displacing the duodenal wall (arrows).
Figure 1: Ultrasound images of the upper abdomen's (a) transverse and (b) sagittal plane. Both images show a hyperechoic mass (asterisks) in the second part of the duodenum. The mass projects into the lumen of the duodenum, anteriorly displacing the duodenal wall (arrows).
Questions
- What is the most likely diagnosis?
- How would you confirm the diagnosis?
- How would you manage this condition?
Answers
- Intramural duodenal hematoma (IDH).
- Computed tomography (CT) or magnetic resonance imaging (MRI) of the abdomen.
- Keep the patient nil per os with gastric decompression and consider total parental nutrition or nasojejunal tube feeding.
Discussion
Congenital factor VII deficiency is a rare bleeding disorder characterized by decreased levels or activity of factor VII, a key component of the coagulation cascade. Its clinical manifestations can range widely from severe life-threatening hemorrhages, such as cerebral, gastrointestinal, and joint hemorrhages, to miscellaneous minor bleeding.1 However, patients with factor VII deficiency do not commonly present with abdominal symptoms, making this case rare and worthy of clinical interest.
Our patient underwent an MRI of the abdomen, which confirmed a lobulated intramural duodenal mass, extending from the second to the third part, causing significant luminal narrowing, consistent with a duodenal hematoma [Figure 2]. He was kept nil per os and commenced on a therapeutic intravenous dose of factor VII and total parenteral nutrition (TPN). The vomiting subsided. After 48 hours, the patient was given a fluid diet, which he tolerated. The oral diet was gradually upgraded, and TPN was withdrawn after a few days. Informed consent was obtained from the patient’s father.
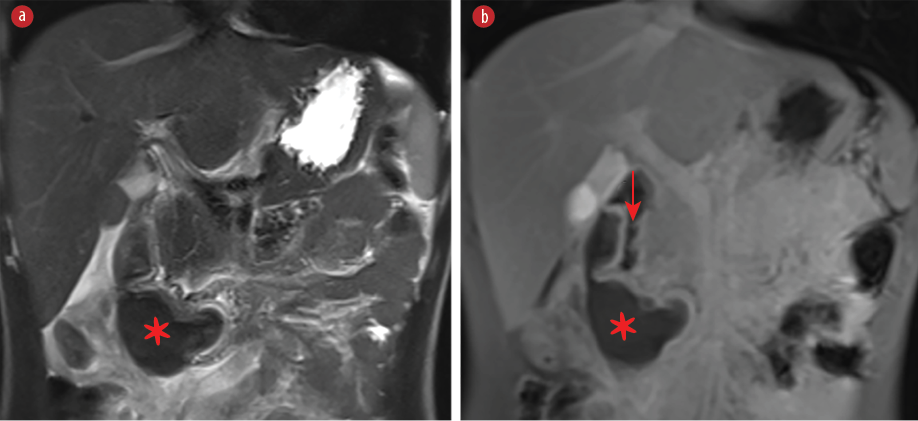 Figure 2: MRI of the abdomen with a coronal T2-weighted image with fat suppression and (b) a contrast-enhanced coronal T1-weighted image with fat suppression shows a lobulated intramural duodenal mass (asterisks) causing significant narrowing of the lumen (arrow). The mass has low T2 signal and shows no enhancement.
Figure 2: MRI of the abdomen with a coronal T2-weighted image with fat suppression and (b) a contrast-enhanced coronal T1-weighted image with fat suppression shows a lobulated intramural duodenal mass (asterisks) causing significant narrowing of the lumen (arrow). The mass has low T2 signal and shows no enhancement.
IDH is a rare condition in children. Blunt trauma to the abdomen is the most common etiology; however, it has been reported to occur spontaneously in patients with coagulation disorders or those on anticoagulant treatment.2 Iatrogenic trauma caused by upper gastrointestinal endoscopy and biopsy has also been reported, especially in children who have undergone hematopoietic stem cell transplantation or with coagulopathy.2,3 However, the occurrence of IDH in patients with inherited coagulopathies is rare.4
Anatomically, the duodenum is divided into four parts and has no mesentery. The first part is located intraperitoneally and the others retroperitoneally. The second and third parts of the duodenum are particularly vulnerable to intramural hematoma due to several factors, including the retroperitoneal position, being close to the spine, the lack of a mesentery, and the rich submucosal vascular supply.3 This was the case with our patient.
The clinical presentation of IDH varies widely from mild and vague abdominal pain to symptoms of complete gastroduodenal obstruction.2 The patients usually complain of abdominal pain, nausea, and vomiting. In children who bleed easily, an IDH can develop and become large enough to cause partial or complete gastroduodenal obstruction.4 Other presentation symptoms include anemia (as in our patient) and acute obstructive pancreatitis secondary to IDH.
A diagnosis of IDH is usually reached by imaging, initially by ultrasound. Abdominal CT scan is considered the gold standard for the confirmatory evaluation of hematoma and related complications like intestinal obstruction, bowel perforation, and obstructive pancreatitis.2,4,5 In the present case, ultrasound suggested IDH and was helpful in monitoring the hematoma. We used an MRI to confirm the diagnosis and exclude complications like pancreatic involvement. MRI was chosen over CT to avoid pediatric radiation risk and because the child was old enough to remain still without anesthesia.
IDH cases are usually managed conservatively with bowel rest, gastric decompression, pain management, and early TPN. Surgery and percutaneous drainage are for patients who fail conservative management and remain symptomatic for over a week.6 Ultrasound/CT-guided drainage or endoscopic incision and drainage have been reported to be successful.3–5 The endoscopic technique involves using a needle-knife or biopsy forceps for the hematoma incision, leading to rapid submucosal decompression. A more recent and increasingly recommended method involves using a lumen-apposing stent such as Hot AXIOS™.6
It is essential to manage the underlying coagulopathy in patients with bleeding disorders.4 Our patient’s factor VII deficiency was treated with recombinant factor VII 30 mcg/kg/dose every three hours until hemostasis was achieved.
Although rare, spontaneous IDH can present as a gastroduodenal obstruction in patients with inherited coagulopathies. The diagnosis requires a high index of suspicion. Most cases can be managed conservatively with bowel rest, TPN, pain management, and correcting the underlying coagulopathy.
Disclosure
The authors declare no conflicts of interest.
references
- 1. Lapecorella M, Mariani G; International Registry on Congenital Factor VII Deficiency. Factor VII deficiency: defining the clinical picture and optimizing therapeutic options. Haemophilia 2008 Nov;14(6):1170-1175.
- 2. Loganathan AK, Bal HS. Intramural duodenal haematoma with mucosal prolapse causing intestinal obstruction. BMJ Case Rep 2019 Apr;12(4):e228276.
- 3. Rahamtalla D, Al Rawahi Y, Al Abri H, Wali Y. Intramural duodenal haematoma in a child post-endoscopic biopsy. BMJ Case Rep 2022 Dec;15(12):e250884.
- 4. Shipp A, Munnikhuysen S, Do A, Radulescu VC. Conservative management of duodenal haematoma in a patient with severe haemophilia: a case report and literature review. Haemophilia 2020 May;26(3):e116-e119.
- 5. Jacobs J, Pekow J. Education and Imaging. Gastroenterology: small bowel obstruction due to multiple spontaneous intramural duodenal hematomas. J Gastroenterol Hepatol 2015 Aug;30(8):1225.
- 6. Valerii G, Ormando VM, Cellini C, Sacco L, Barbera C. Endoscopic management of intramural spontaneous duodenal hematoma: a case report. World J Gastroenterol 2022 May;28(20):2243-2247.