Urinary tract infections (UTIs) are the second most prevalent infectious ailment worldwide, affecting over 150 million individuals annually.1 The reported prevalence of UTI among adults and children in the Arabian Gulf Cooperation Council (GCC) countries ranges between 18.6% and 68.7%.2
Diagnosing and managing UTI is a critical and challenging aspect of clinical practice.3 Presentations range from asymptomatic bacteriuria (ASB) to pyelonephritis. Symptoms commonly manifest as dysuria, frequency and urgency to urinate, suprapubic pain, and occasionally hematuria. Asymptomatic UTIs frequently occur among vulnerable populations such as the elderly, pregnant women, and patients with underlying genitourinary conditions or catheterization. Due to a lack of typical UTI symptoms, asymptomatic cases are more challenging to diagnose.4
Accurate diagnosis of UTIs remains essential for guiding effective treatment and combating antibiotic resistance.5 While urinalysis is an initial screening tool, culture and sensitivity testing ensures precision, which is essential for choosing optimal treatment.6
However, knowledge gaps persist regarding the necessity of treatment for symptomatic cases, regardless of white blood cell (WBC) presence in the urine, and in establishing connections between symptoms, urine WBC counts, culture outcomes, and the presence of multidrug-resistant (MDR) organisms. The rising global prevalence of MDR pathogens emphasizes the need to monitor local epidemiological and resistance patterns to guide treatment and antibiotic stewardship. This is particularly important for the GCC, with its multiethnic and dynamic population.
Therefore, the primary objectives of this study, based on a GCC population, are twofold: first, to establish the necessity of the treatment of symptomatic and asymptomatic UTI cases, regardless of the presence of WBCs on routine urine or culture growth; and second, to align treatment with the most sensitive and administrable first-line antibiotic, based on culture reports. Our secondary objectives were to correlate symptoms with urine WBC ranges detected in routine and culture tests, identify the most common urinary pathogen detected in our institution, determine the most-prescribed empiric first-line antibiotic before culture reports, and quantify the prevalence of MDR organisms.
Methods
This retrospective observational study was conducted at a private tertiary hospital in Muscat. Electronic medical records of urology patients of both sexes, aged seven months to 81 years, who attended the hospital between January and July 2023, were reviewed. The study population included Omani nationals and expatriate residents, primarily from Sudan, India, Pakistan, and Bangladesh. The Medical Director’s permission was duly obtained for using the patient data on the hospital electronic medical records with maintained confidentiality.
Data of 223 patients were obtained, a sample size comparable to that used in a prior Iranian study by Ziaei et al,7 which included 200 patients. The patient cohort included outpatients and inpatients, and critical cases from the emergency department and the intensive care unit. Cases with bacterial growth on urine culture were included, while those with Candida sp. or incomplete information were excluded.
Collected data included demographic information (including date of presentation, age, sex, and admission status), clinical symptoms, urine routine WBC counts, cultured organisms, antibiotic sensitivity patterns, and MDR status. Information regarding the most sensitive and first-line antibiotics, along with their minimum inhibitory concentration (MIC) values, prescribed antibiotics, and follow up status, was also recorded.
All cases were managed in accordance with our hospital procedure, based on established clinical protocols. For symptomatic UTI cases, an empiric antibiotic regimen (first line of treatment) was initially prescribed based on the patient’s presentation symptoms and clinical history, and the urine samples were sent for culture and sensitivity testing to determine the microbial etiology and susceptibility to various antibiotics. Based on the report, the patient’s antibiotic prescription was revised if necessary, prioritizing the use of the most sensitive antibiotic MIC1 identified in the report. However, if the preferred antibiotic MIC1 required parenteral administration and the patient could tolerate oral therapy, the second most sensitive antibiotic MIC2 available for oral administration was prescribed, ensuring optimal management aligned with each patient’s clinical needs and microbiological profile.
Urine specimens were collected aseptically via midstream collection, catheterization, or suprapubic aspiration. Patients were instructed to provide clean-catch samples to minimize contamination.
Urine samples were microbiologically processed for a routine test and microscopic examination and cultured on blood agar and CLED agar plates (Oxoid, UK). The plates were incubated for 24 hours at 37 °C. Single colonies were isolated from samples with significant growth. Microbial identification was performed using the automated VITEK2 System (bioMerieux). UTI was diagnosed when significant growth (≥ 105 colony-forming units/mL) of one or more organism/s was observed. Samples showing zero to insignificant growth in 24 hours were incubated for another 24 hours, and the procedure was repeated.
For statistical analysis, we used R i386 Core Team, version 3.6.3 and Microsoft Excel. Continuous variables were represented by mean and SD and categorical variables using frequency tables. Kruskal-Wallis test and Mann-Whitney U test with Bonferroni adjustment were used for non-normal continuous variables. The Shapiro-Wilk test was used to assess normality. Categorical data was compared using the chi-square test. A p-value < 0.05 was considered statistically significant.
Results
A total of 223 cases were included in the study. The average age of patients was 36.3 ± 17.6 years, with females accounting for 83.4%. Symptomatic UTIs were reported by 85.7% of the cohort [Table 1]. Even though 85.0% of females had symptomatic UTI, the distribution difference based on sex lacked statistical significance. Symptomatic patients had a higher mean age (37.6 years), a statistically significant difference (p = 0.009).
Table 1: Characteristics of patients by demographic details (N = 223).
|
Age group , years (overall mean age: 36.4 ± 17.6 years)
|
|
0–10
|
24 (10.8)
|
|
11–20
|
8 (3.6)
|
|
21–30
|
45 (20.2)
|
|
31–40
|
73 (32.7)
|
|
41–50
|
27 (12.1)
|
|
51–60
|
24 (10.8)
|
|
61–70
|
13 (5.8)
|
|
71–81
|
9 (4.0)
|
|
Sex
|
|
|
Male
|
37 (16.6)
|
|
Female
|
186 (83.4)
|
|
Presentation
|
|
|
Symptomatic
|
191 (85.7)
|
|
Asymptomatic
|
32 (14.3)
|
|
Age group distribution among males, years
|
|
0–10
|
7 (18.9)
|
|
11–20
|
1 (2.7)
|
|
21–30
|
1 (2.7)
|
|
31–40
|
13 (35.1)
|
|
41–50
|
2 (5.4)
|
|
51–60
|
5 (13.5)
|
|
61–70
|
5 (13.5)
|
|
71–81
|
3 (8.1)
|
|
Age group distribution among females, years
|
|
0–10
|
17 (9.1)
|
|
11–20
|
5 (2.7)
|
|
21–30
|
47 (25.3)
|
|
31–40
|
58 (31.2)
|
|
41–50
|
26 (14.0)
|
|
51–60
|
19 (10.2)
|
|
61–70
|
8 (4.3)
|
Older patients (37.6 ± 17.1) were significantly more likely to be symptomatic than younger patients (29.0 ± 18.6 years); p = 0.009), but there was no significant difference between sexes in this respect, Although females had higher UTI prevalence than males within both symptomatic and asymptomatic groups (82.7% vs. 17.3%, and 87.5% vs. 12.5%, respectively), this difference was not significant. Symptomatic cases were far more prevalent than asymptomatic ones in both groups (89.2% vs. 10.8% and 84.9% vs. 15.1%, for both males and females, respectively) [Table 2].
Table 2: Comparison of demographic data stratified by the type of urinary tract infection (N = 223).
|
Age, years, mean ± SD
|
37.6 ± 17.1
|
29.0 ± 18.6
|
0.009 M*
|
|
Sex**
|
|
|
|
|
Male
|
33 (89.2)
|
4 (10.8)
|
0.677C#
|
M: Mann-Whitney U test; C: chi-square test.
*Significant; **% is calculated based on column count; #The proportion is not equal in the group.
Table 3 describes the variations of WBC in urine. Over one-third (37.7%) of symptomatic patients had WBC levels of 3+, while 29.3% had ‘nil’ WBC levels. In contrast, 53.1% of asymptomatic patients had ‘nil’ WBC, with significant differences between the two groups (p = 0.014). Additionally, while there was no significant difference in the distribution of WBC levels for 1+, 2+,3+, and 4+ categories, a notable trend was obeserved with a higher percentage of WBC (combination of 3+ and 4+) in the symptomatic group (42.4%) compared to asymptomatic group (21.9%). In addition, distributions of urine white blood cells were significantly different between patients with symptomatic and asymptomatic UTI
(p < 0.001) [Table 3].
Table 3: Comparison of demographic data according to the presence of WBC in urine (N = 223).
|
Urine WBC**
|
|
|
|
|
NIL
|
56 (29.3)
|
17 (53.1)
|
0.014*C
|
|
1+
|
23 (12.0)
|
4 (12.5)
|
> 0.990CS
|
|
2+
|
31 (16.2)
|
4 (12.5)
|
0.784CS
|
|
3+
|
72 (37.7)
|
6 (18.8)
|
0.060C
|
|
4+
|
9 (4.7)
|
1 (3.1)
|
> 0.990CS
|
WBC: white blood cell (** % calculated based on raw total); C: chi-square test; CS: chi-square test with simulation.
*Significant.
Escherichia coli (E. coli) was the most isolated organism (55.6%) in our study. Other primary isolates were Streptococcus agalactiae (S. agalactiae), Klebsiella pneumoniae (K. pneumoniae), and Enterococcus faecalis (E. faecalis). Interestingly, E. faecalis was more prevalent in asymptomatic cases (12.5%) than symptomatic cases (3.7%). Staphylococcus saprophyticus (S. saprophyticus) and Staphylococcus haemolyticus (S. haemolyticus) were isolated only in symptomatic cases [Figure 1].
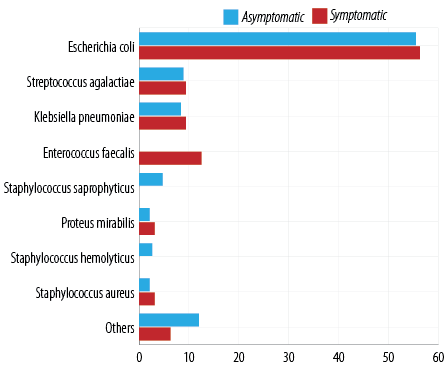 Figure 1: Distribution of symptomatic and asymptomatic patients stratified by pathogens identified (N = 223).
Figure 1: Distribution of symptomatic and asymptomatic patients stratified by pathogens identified (N = 223).
For symptomatic UTI, levofloxacin and nitrofurantoin emerged as the most frequently prescribed first-line antibiotic, followed by amoxicillin-clavulanate (Augmentin). For asymptomatic cases, either Augmentin or ceftriaxone was the preferred first-line antibiotic (15.6% each). In the symptomatic group, 82.2% of patients received treatment, against 71.9% in the asymptomatic group (second-line of antibiotic treatment based on MIC 1 results) [Table 4].
Table 4: Distribution of symptomatic and asymptomatic patients by empirically prescribed antibiotics (N = 223).
|
No antibiotic
|
34 (17.8)
|
9 (28.1)
|
|
Levofloxacin
|
29 (15.2)
|
2 (6.3)
|
|
Nitrofurantoin
|
28 (14.7)
|
2 (6.3)
|
|
Amoxicillin-clavulanate (Augmentin®)
|
20 (10.5)
|
5 (15.6)
|
|
Cefixime
|
16 (8.4)
|
4 (12.5)
|
|
Ceftriaxone
|
10 (5.2)
|
5 (15.6)
|
|
Cefuroxime
|
10 (4.2)
|
2 (9.4)
|
|
Ciprofloxacin
|
11 (5.8)
|
0 (0.0)
|
|
Cefditoren
|
6 (3.1)
|
2 (6.3)
|
|
Cefdinir
|
7 (3.7)
|
0 (0.0)
|
|
Fosfomycin (Fosfolag) sachet
|
6 (3.1)
|
0 (0.0)
|
The totals may exceed 100% as some patients were prescribed multiple antibiotics.*Cotrimoxazole, gentamicin, cefixime, meropenem, amikacin, etc.
Table 5 illustrates the distribution of cases by antibiotic sensitivity (most sensitive as per the culture report) among symptomatic and asymptomatic groups. Nitrofurantoin showed susceptibility in 144 (75.4%) symptomatic cases and in 25 (78.1%) asymptomatic cases. The second most sensitive antibiotic was ciprofloxacin in both symptomatic and asymptomatic cases (33.0% and 25.0%, respectively).
Table 5: Distribution of symptomatic and asymptomatic patients by antibiotic sensitivity (first line antibiotics as per the culture report) (N = 223).
|
Nitrofurantoin
|
144 (75.4)
|
25 (78.1)
|
|
Ciprofloxacin
|
63 (33.0)
|
8 (25.0)
|
|
Cefepime
|
29 (15.2)
|
2 (6.3)
|
|
Meropenem
|
21 (11.0)
|
1 (3.1)
|
|
Augmentin/ Amoxiclav
|
19 (10.0)
|
4 (12.5)
|
|
Levofloxacin
|
14 (7.3)
|
4 (12.5)
|
|
Moxifloxacin
|
11 (5.8)
|
2 (6.3)
|
|
Ampicillin
|
8 (4.2)
|
1 (3.1)
|
|
Gentamicin
|
6 (3.1)
|
2 (6.3)
|
|
Imipenem
|
7 (3.7)
|
1 (3.1)
|
The total may exceed 100% as several patients were prescribed multiple antibiotics.
Table 6 compares the presence of MDR bacteria and follow-up cultures in all UTI cases. Specifically, there was no significant difference in the prevalence of MDR bacteria between symptomatic and asymptomatic groups (p = 0.377). Similarly, the presence of positive follow-up cultures did not show a significant association with UTI type (p = 0.687). These findings imply that the presence of MDR bacteria and the need for subsequent cultures may not be influenced by the symptomatic manifestation of UTI, regardless of whether symptoms are present or absent.
Table 6: Association of MDR and follow-up culture with symptomatic and asymptomatic types of urinary tract infection (N = 223).
|
MDR
|
Yes
|
9 (4.7)
|
0 (0.0)
|
9 (4.0)
|
0.377CS
|
|
No
|
182 (95.3)
|
32 (100)
|
214 (96.0)
|
|
|
Follow up culture
|
Yes
|
12 (6.3)
|
3 (9.4)
|
15 (6.7)
|
0.687CS
|
MDR: multidrug-resistance; CS: chi-square test with simulation.
Nitrofurantoin and levofloxacin were the most prescribed empirical antibiotics in both symptomatic and asymptomatic cases [Table 4], and nitrofurantoin confirmed its highest sensitivity in MIC1 [Figure 2]. Levofloxacin was the most frequently prescribed antibiotic for nitrofurantoin-sensitive cases, followed by Augmentin. The clinical indication of glucose-6-phosphate dehydrogenase deficiency and the availability of nitrofurantoin (only one brand available in Oman) may have been the factors in choosing the above antibiotic. In nitrofurantoin-sensitive cases, irrespective of prescribed antibiotics, a consistent median MIC1 of 16 µg/mL was observed, indicating uniform sensitivity to nitrofurantoin across both groups [Figure 2].
MIC: Minimum inhibitory concentration.
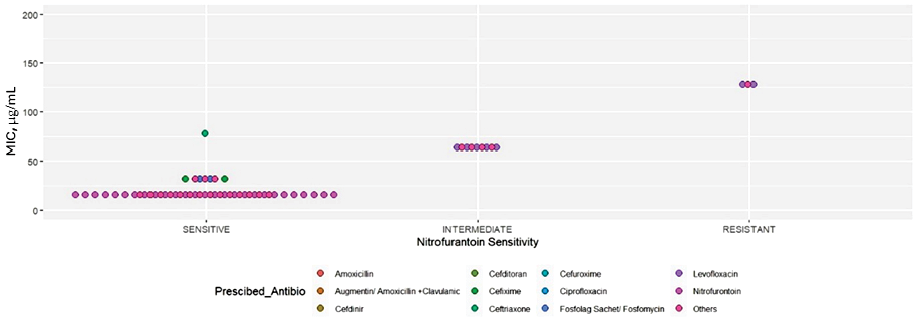 Figure 2: Distribution of MIC by prescribed drugs for the isolates based on sensitivity to nitrofurantoin.
Figure 2: Distribution of MIC by prescribed drugs for the isolates based on sensitivity to nitrofurantoin.
Discussion
This retrospective observational study investigated the clinical and microbiological factors influencing UTI management in a tertiary healthcare setting in Oman. The participants included urology patients suffering from both uncomplicated and complicated UTI, and the investigation focused on the management of asymptomatic, symptomatic, and recurrent forms of the condition, diagnosed using urine cultures that were also tested for antibiotic susceptibility. The management focused on preventing urological complications such as pyelonephritis, renal scarring, and bladder dysfunction.
Females comprised the majority (83.4%) of our participants, aligning with the literature that attributes the higher UTI prevalence among women to anatomical factors, particularly the shorter female urethra, which facilitates easier bacterial entry into the urinary system.8,9 Bhargava et al,10 in Northern India and Mitu et al,11 in Bangladesh also found higher UTI prevalence among females (60.7% and 83.5%, respectively). An extensive Nigerian study with 1120 patients also noted a greater burden of UTIs in females (56.3%).12 In the GCC region, a systematic review of 58 Saudi Arabian studies reported a female predominance (66.7%) in UTI prevalence.13
Our female participants aged 31–40 years had the highest incidence of UTI among all age groups and both sexes. Unexpectedly, we observed a relatively lower rate of UTI in our older participants, especially those aged 51–81 years, whose prevalence was comparable to those in the pediatric population. This is contrary to the findings elsewhere, where older people had a higher prevalence of UTI. The reason for the contrary results in our study is unclear; however, one possible explanation is that more than 50% of our participants were in the 21–40 age group, which may have skewed the data towards younger ages. Therefore, we should not overlook the older age group of women, as UTIs pose a substantial risk among elderly women, ranking as the second most common infection in this demographic.14,15 It represents the primary cause of infection in hospitalized elderly women or those residing in long-term care facilities.16 A UK study conducted from 2004 to 2014 among patients aged ≥ 65 years found that the incidence of UTI increased with age. This trend was higher among women, at 9–11 cases per 100 person-years in the 65–74 age group, 11.4–14.3 in the 75–84 age group, and 14.7–19.8 in those > 85. Corresponding rates among men were much lower (2.8–3.0, 5.9–6.1, and 8.1–10.5, respectively).
Our patients with symptomatic UTI were significantly older than those with asymptomatic UTI, which is supported by previous studies. The higher mean age in symptomatic cases may indicate the impact of age-related cumulative factors such as urine retention, incontinence, and other comorbidities that predispose older people to symptomatic infections.17
UTIs among older adults may not always manifest with classic symptoms; vague indicators like confusion or lethargy may be present.15 Postmenopausal women, due to estrogen deficiency, may face an increased UTI risk. In contrast, the risk factors in older men tend to be bladder or kidney stones, enlarged prostate, catheter use, and bacterial prostatitis.5,16 Recurrent UTIs impose a significant social and economic burden, negatively affecting quality of life and burdening healthcare systems. Risk factors in the elderly also include age-related immune changes, exposure to nosocomial pathogens, multiple comorbidities, and prior UTI history.16
In our study, symptomatic UTI cases were significantly more prevalent than asymptomatic ones, with similar distribution patterns observed in both sexes. Although there was a trend of higher urine WBC levels (3+ and 4+) in symptomatic cases versus asymptomatic ones, the correlation was not significant, indicating that WBC levels are insufficient for distinguishing between the two conditions.18 Given the diagnostic limitations of urine WBC levels, it is essential to conduct a comprehensive clinical assessment and urine culture with antibiotic sensitivity.
The distribution patterns of isolated organisms in this study population provided valuable insights into the microbial etiology of UTI. E. coli emerged as the predominant pathogen, aligning with its well-established global role as the leading cause of UTIs.19,20 Other common pathogens included S. agalactiae, K. pneumoniae, and E. faecalis, reflecting the diversity of microbial flora associated with UTI. We also identified differences in microbial prevalence between symptomatic and asymptomatic cases, with E. faecalis exhibiting a higher prevalence in asymptomatic cases, which is concerning due to its global significance as a causative agent of UTI.
Previous studies have documented E. coli and K. pneumoniae as the primary etiological agents responsible for complicated UTI cases.21–24 This can be explained by the presence of diverse virulence factors that augment their capacity to colonize the urinary tract and evade the host immune system. Among these factors are adhesins that facilitate binding to the uroepithelial cells, toxins that damage host tissues, and mechanisms to evade phagocytosis by immune cells. These result in resistance toward broad-spectrum penicillins, third-generation cephalosporins (cefotaxime, ceftriaxone, and ceftazidime), fourth-generation cephalosporin (cefepime), gentamicin, and old fluoroquinolones.23,24 Urinary catheters are known to significantly increase the risk of infection by E. coli and K. pneumoniae, as these devices can provide a direct pathway for bacteria to enter the bladder, causing urological complaints of varying severity, depending on the pathogen.25
No case of ASB was detected in the current study; however, they need to be included in the differential diagnosis. ASB is diagnosed when a urine culture indicates a significant bacterial count (usually defined as ≥105 colony-forming units per mL) in the absence of UTI symptoms such as dysuria, increased urinary frequency, or fever.26 Treating ASB with antibiotics has long been known to be counterproductive. In the 1980s, a Canadian study randomized 50 elderly institutionalized women with ASB to receive either antimicrobial therapy for all instances of ASB or no treatment unless UTI symptoms emerged. During the one-year study period, the women who received antimicrobials had a lower prevalence of bacteriuria but experienced more reinfections and adverse drug reactions.27 In 2015, Cai et al,28 investigated the effects of antibiotic treatment among Italian women with recurrent UTIs. Patients who received antibiotics had higher recurrence rates and increased antibiotic resistance in E. coli isolates compared to those who did not receive antibiotic.
These findings suggest that treating ASB cases may be ineffective and counterproductive due to higher recurrence rates and risk of antibiotic resistance, underscoring the importance of differentiating between cases of symptomatic UTI, asymptomatic UTI, and ASB, and adopting evidence-based approaches for each condition.28
The high prevalence of E. faecalis in asymptomatic UTIs in our study warrants further investigation into management strategies and implications. The microbial composition and antibiotic resistance patterns in asymptomatic UTIs, especially those with high E. faecalis prevalence, warrant further research and clinical insights to provide targeted therapy while minimizing antibiotic resistance.
The association between MDR prevalence and UTI type in our study yielded interesting findings, although they were not statistically significant. While MDR pathogens were detected in a minority of symptomatic cases, none was identified among asymptomatic cases, again without significance.
The antibiotic sensitivity patterns revealed by our MIC analysis highlight the importance of accurate MIC assessment for effective infection treatment. Elevated MIC levels near breakpoints may indicate early signs of resistance and potential therapy failure. Understanding the susceptibility of bacterial strains to typical antibiotics used in hospitals and communities will aid in antibiotic stewardship efforts.29 Furthermore, the efficacy of powerful antibiotics like meropenem against MDR E. coli strains highlights the necessity of reserving such potent drugs for complicated or resistant UTI cases.
Our MIC1 results identified nitrofurantoin as the most sensitive antibiotic, justifying our choice of this drug as the first-line of therapy for UTI. A recent study from Singapore reported that 99.4% of otherwise-resistant E coli isolates were susceptible to nitrofurantoin in female patients with uncomplicated UTI.30 Additionally, an American retrospective study reported a 93.3% success rate with nitrofurantoin therapy.31 Nitrofurantoin’s efficacy in otherwise resistant pathogens, favorable safety profile, and broad-spectrum activity highlight its suitability as a first-line treatment for uncomplicated UTIs.32 A Saudi Arabian study also found nitrofurantoin to be the most effective antibiotic with the lowest cost for uncomplicated UTIs.33
Frequently, levofloxacin is used to treat nitrofurantoin-sensitive UTI cases. Levofloxacin is a broad-spectrum antibiotic that is effective against a wide range of gram-positive and gram-negative bacteria commonly implicated in UTIs. Levofloxacin may be prescribed where nitrofurantoin is contraindicated or ineffective, such as in patients with known allergies, kidney impairment, or resistance to nitrofurantoin. Additionally, levofloxacin offers the advantage of once-daily dosing, which facilitate better patient compliance and potentially reduce the risk of antibiotic resistance.34,35
We also identified a few antibiotics with low effectiveness in treating UTI, such as cefepime, meropenem, and gentamicin, emphasizing the importance of judicious antibiotic selection guided by local susceptibility patterns and antimicrobial stewardship principles.36
The strengths of the present study include its relatively large sample size, comprising intensive care unit, outpatient department, and emergency patients, which enabled the inclusion of uncomplicated, complicated, and recurrent cases. The study highlights the clinical profile and treatment approach to UTIs in the GCC region including Oman, emphasizing its importance given the region’s distinct clinical and epidemiological landscape. This study emphasizes the importance of urine cultures and susceptibility testing in guiding antibiotic therapy for complicated or recurrent UTIs, while also advocating for judicious antibiotic use to combat antimicrobial resistance, especially amid rising MDR prevalence.
The limitations of the study include its single-center setting, retrospective design with its characteristic limitations, such as the absence of clinical outcome data, and a lack of distinction between complicated and uncomplicated UTIs. Future research should adopt prospective, multicenter approaches to enhance generalizability and comprehensively evaluate UTI management efficacy. In addition, being from a privately run institution, the current sample included a larger proportion of non-Omanis, especially from South Asian countries, than in typical studies from Oman, which has the potential to skew regional comparisons. Thus, a comparison of our results with other similar studies from Oman should be made with caution.
Emerging diagnostic technologies, such as rapid molecular diagnostics, enable faster and more accurate identification of UTI pathogens.37 These technologies need to be more widely available to facilitate prompt and targeted antibiotic treatment to UTI patients in the GCC countries including Oman. Addressing challenges in obtaining diagnostic reports is crucial for enhancing clinical decision-making and mitigating suboptimal UTI management practices. Further studies are warranted to delineate the necessity of treatment and identify optimal antibiotic regimens, fostering more targeted and efficacious UTI interventions.
Conclusion
The present study revealed that patient age is a key factor in UTI management among patients attending a privately run hospital in Oman. E. coli was the predominant pathogen in urine cultures and nitrofurantoin is the most effective first-line antibiotic. Our results emphasize the importance of follow up care for patients with multidrug-resistant UTI to prevent complications. Customized antibiotic therapy based on culture and sensitivity results is crucial for optimizing treatment outcomes and combating antimicrobial resistance. Future research should focus on prospective multicenter studies to confirm these findings, explore new therapeutic approaches, and antimicrobial stewardship strategies.
Disclosure
The authors declare no conflicts of interest. No funding was received for this study.
Acknowledgment
We sincerely thank Ashraf, Jisha, Renjini, Shobhita, Sreepriya, Bindu, Akhila, and Arya from the lab team for their assistance in data collection, and Dr. Neesha Nair for initiating and supporting the project.
references
- 1. Mlugu EM, Mohamedi JA, Sangeda RZ, Mwambete KD. Prevalence of urinary tract infection and antimicrobial resistance patterns of uropathogens with biofilm forming capacity among outpatients in Morogoro, Tanzania: a cross-sectional study. BMC Infect Dis 2023 Oct;23(1):660.
- 2. Albarrak M, Al Dabbagh M, Al Hashami H, Alzomor O, Ghatasheh G, Habashy N, et al. Urinary tract infections in children from the Gulf Cooperation Council countries: a literature review (2011-2022). Front Pediatr 2023 Jul;11:1163103.
- 3. Marantidis J, Sussman RD. Unmet needs in complicated urinary tract infections: challenges, recommendations, and emerging treatment pathways. Infect Drug Resist 2023 Mar;16:1391-1405.
- 4. Sabih A, Leslie SW. Complicated urinary tract infections. StatPearls. 2023 [cited 2024 June 13]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK436013/.
- 5. Davenport M, Mach KE, Shortliffe LM, Banaei N, Wang TH, Liao JC. New and developing diagnostic technologies for urinary tract infections. Nat Rev Urol 2017 May;14(5):296-310.
- 6. MacNeill AL, Barger AM. Clinical pathology and laboratory techniques for veterinary technicians. 2nd ed. 2023. p. 181-218.
- 7. Ziaei S, Ninavaei M, Faghihzadeh S. Urinary tract infection in the users of depot-medroxyprogesterone acetate. Acta Obstet Gynecol Scand 2004 Oct;83(10):909-911.
- 8. Czajkowski K, Broś-Konopielko M, Teliga-Czajkowska J. Urinary tract infection in women. Prz Menopauzalny 2021 Apr;20(1):40-47.
- 9. Jagtap S, Harikumar S, Vinayagamoorthy V, Mukhopadhyay S, Dongre A. Comprehensive assessment of holding urine as a behavioral risk factor for UTI in women and reasons for delayed voiding. BMC Infect Dis 2022 Jun;22(1):521.
- 10. Bhargava K, Nath G, Bhargava A, Kumari R, Aseri GK, Jain N. Bacterial profile and antibiotic susceptibility pattern of uropathogens causing urinary tract infection in the eastern part of Northern India. Front Microbiol 2022 Aug;13:965053.
- 11. Mitu FS, Al Maruf MA, Mahanty A, Huda AK, Khan SA, Rahman MM. Prevalence of extended spectrum beta-lactamase (ESBL) and AmpC beta-lactamase producing bacteria in urinary tract infection patients in Bangladesh. Malays J Microbiol 2019;15(3):204-212.
- 12. Nuhu T. Retrospective studies on the prevalence of uropathogens in Sokoto metropolis. Afr J Microbiol Res 2015;9(20):1366-1370.
- 13. Sula I, Alreshidi MA, Alnasr N, Hassaneen AM, Saquib N. Urinary tract infections in the Kingdom of Saudi Arabia, a review. Microorganisms 2023 Apr;11(4):952.
- 14. Thangrom W, Roopsawang I, Aree-Ue S. Prevalence and related factors of lower urinary tract infection in frail older adults undergoing major noncardiac surgery. Geriatrics (Basel) 2023 Feb;8(2):33.
- 15. Rodriguez-Mañas L. Urinary tract infections in the elderly: a review of disease characteristics and current treatment options. Drugs Context 2020 Jul;9(Jul):9.
- 16. Rowe TA, Juthani-Mehta M. Urinary tract infection in older adults. Aging health 2013 Oct;9(5):519-528.
- 17. Cortes-Penfield NW, Trautner BW, Jump RLP. Urinary tract infection and asymptomatic bacteriuria in older adults. Infect Dis Clin North Am 2017 Dec;31(4):673-688.
- 18. N. Givler D. Givler A. Asymptomatic bacteriuria. StatPearls. 2023 [cited 2024 June 13]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441848/.
- 19. Zagaglia C, Ammendolia MG, Maurizi L, Nicoletti M, Longhi C. Urinary tract infections caused by uropathogenic escherichia coli strains-new strategies for an old pathogen. Microorganisms 2022 Jul;10(7):1425.
- 20. Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med 2002 Jul;113(1)(Suppl 1A):14S-19S.
- 21. Kaye KS, Belley A, Barth p, Lahlou O, Knechtle p, Motta p, et al. Effect of Cefepime/Enmetazobactam vs Piperacillin/Tazobactam on clinical cure and microbiological eradication in patients with complicated urinary tract infection or acute pyelonephritis: a randomized clinical trial. JAMA 2022 Oct;328(13):1304-1314.
- 22. McAteer J, Lee JH, Cosgrove SE, Dzintars K, Fiawoo S, Heil EL, et al. Defining the optimal duration of therapy for hospitalized patients with complicated urinary tract infections and associated bacteremia. Clin Infect Dis 2023 May;76(9):1604-1612.
- 23. Rouphael N, Winokur p, Keefer MC, Traenkner J, Drobeniuc A, Doi Y, et al; DMID 15-0045 study group. Daily fosfomycin versus levofloxacin for complicated urinary tract infections. mBio 2023 Oct;14(5):e0167723.
- 24. Li Y, Yin Y, Peng X, Zheng H, Fu F, Liu Z, et al. A randomized, active-controlled, multicentre clinical trial to evaluate the efficacy and safety of oral sitafloxacin versus levofloxacin in Chinese adults with acute uncomplicated or complicated urinary tract infection. Ann Med 2021 Dec;53(1):217-226.
- 25. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015 May;13(5):269-284.
- 26. Trautner BW, Grigoryan L. Approach to a positive urine culture in a patient without urinary symptoms. Infect Dis Clin North Am. 2014 Mar;28(1):15-31.
- 27. Nicolle LE, Mayhew WJ, Bryan L. Prospective randomized comparison of therapy and no therapy for asymptomatic bacteriuria in institutionalized elderly women. Am J Med 1987 Jul;83(1):27-33.
- 28. Cai T, Nesi G, Mazzoli S, Meacci F, Lanzafame p, Caciagli p, et al. Asymptomatic bacteriuria treatment is associated with a higher prevalence of antibiotic resistant strains in women with urinary tract infections. Clin Infect Dis 2015 Dec;61(11):1655-1661.
- 29. Kowalska-Krochmal B, Dudek-Wicher R. The minimum inhibitory concentration of antibiotics: methods, interpretation, clinical relevance. Pathogens 2021 Feb;10(2):1-21.
- 30. Croom KF, Goa KL. Levofloxacin: a review of its use in the treatment of bacterial infections in the United States. Drugs 2003;63(24):2769-2802.
- 31. Ghouri F, Hollywood A. Antibiotic prescribing in primary care for urinary tract infections (UTIs) in pregnancy: an audit study. Med Sci (Basel) 2020 Sep;8(3):40.
- 32. Koh SW, Ng TS, Loh VW, Goh JC, Low SH, Tan WZ, et al. Antibiotic treatment failure of uncomplicated urinary tract infections in primary care. Antimicrob Resist Infect Control 2023 Aug;12(1):73.
- 33. Zilberberg MD, Nathanson BH, Sulham K, Shorr AF. Antimicrobial susceptibility and cross-resistance patterns among common complicated urinary tract infections in U.S. hospitals, 2013 to 2018. Antimicrob Agents Chemother 2020 Jul;64(8):e00346-e20.
- 34. Huttner A, Verhaegh EM, Harbarth S, Muller AE, Theuretzbacher U, Mouton JW. Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials. J Antimicrob Chemother 2015 Sep;70(9):2456-2464.
- 35. Alanazi MQ. Clinical efficacy and cost analysis of antibiotics for treatment of uncomplicated urinary tract infections in the emergency department of a tertiary hospital in Saudi Arabia. Ther Clin Risk Manag 2021 Nov;17:1209-1217.
- 36. Patel SJ, Saiman L. Principles and strategies of antimicrobial stewardship in the neonatal intensive care unit. Semin Perinatol 2012 Dec;36(6):431-436.
- 37. Messacar K, Parker SK, Todd JK, Dominguez SR. Implementation of rapid molecular infectious disease diagnostics: the role of diagnostic and antimicrobial stewardship. J Clin Microbiol 2017 Mar;55(3):715-723.