Beckwith-Wiedemann syndrome (BWS, OMIM #130650) is a rare genomic imprinting disorder and the most common overgrowth syndrome characterized by complex of clinical and molecular features.1,2 It is now considered a BW spectrum (BWSp) that encompasses both classic form of BWS and lateralized overgrowth (OMIM #235000) initially termed as isolated hemihypertrophy (IHH) [Figure 1].3
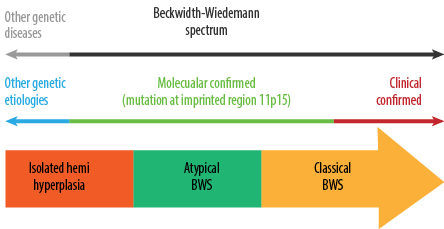 Figure 1: Beckwith–Wiedemann spectrum (BWSp); classical and atypical BW syndrome and isolated lateralized overgrowth.
Figure 1: Beckwith–Wiedemann spectrum (BWSp); classical and atypical BW syndrome and isolated lateralized overgrowth.
Clinicians and researchers distinguish between two categories of clinical features observed in patients with BWS. Cardinal features are crucial for confirming diagnoses, while suggestive features, more prevalent in the general pediatric population, have a lesser impact on the scoring system.
Genetic alterations on chromosome region 11p15.5 are considered to be the main cause of BWS, accounting for 80% of cases, with DNA methylation as the most common molecular abnormaliy.4–6 Gain of methylation at H19/IGF2: IG (DMR; impriniting control (IC) 1 GOM), loss of methylation at KCNQ1OT1: TSS (DMR; IC2 LOM), paternal uniparental isodisomy (pUPD11), CDKN1C mutation, and cytogenetic abnormalities have been identified in BWS.7
Embryonal tumors are seen in about 8% of BWSp children, with Wilms tumor (52%), hepatoblastoma (14%), neuroblastoma (10%), rhabdomyosarcoma (5%), and adrenal carcinoma (3%) being the most prevalent.8 Therefore, tumor surveillance strategies play a central role in managing and following-up with affected patients to increase survival and reduce mortality.3 Regular tumor surveillance is generally advised for all BWS patients to detect the two most common tumors, Wilms tumor and hepatoblastoma, through serial alpha-fetoprotein testing and abdominal/renal ultrasounds.9
The clinical and molecular characteristics of BWS in European and North American patients have been extensively studied, and some studies have been also conducted in China.3,10 Few studies have investigated the potential (epi)genotype-phenotype connections.11,12 Some highlighted the tumor risk in BWSp population.3 However, no studies were conducted on Omani patients with BWSp or their risk of cancer.
This study aimed to characterize Omani patients with BWS and IHH clinically and molecularly, evaluate their surveillance results, and assess tumor prevalence in the cohort.
Methods
This retrospective cohort study examined the clinical and molecular characteristics of patients with BWS and IHH who attended the genetic clinic at Sultan Qaboos University Hospital (SQUH) over the past decade. The study was ethically approved by the Medical Research Ethical Committee in Sultan Qaboos University (MREC # 2834).
Nine children with clinically confirmed BWS and IHH who attended SQUH between January 2012 and December 2022 were included in the study. All patients with BWS who fulfilled the scoring criteria and those with IHH were included. The scoring system assigned two points for each cardinal feature and one point for each suggestive feature. Patients with a BWS score of ≥ 4 met the clinical diagnostic criteria for classical BWS.3
Data were gathered from the electronic patient records, including demographic information (age and gender), initial anthropometric measurements (height in cm, weight in kg, and head circumference in cm), as well as subsequent measurements from at least two follow up visits. Data also included consanguinity, family history of similar presentations or cancer, clinical findings, and results from methylation tests. Methylation-specific-multiplex ligation-dependent probe amplification (MS-MLPA) was used in all patients to confirm the molecular pathology. This MS-MLPA can simultaneously detect methylation and copy number status (deletion or duplication) of the differentially methylated regions related to BWS at 11p15. Additionally, AFP test results, ultrasound (US) imaging results, and other relevant radiological results such as magnetic resonance imaging (MRI), computed tomography, and X-ray were compiled.
Statistical analyses were performed using SPSS (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp), including descriptive statistics and Fisher’s exact test for associations between clinical features and either BWS or IHH. Statistical significance was set at p < 0.05.
Results
The study included nine patients with BWSp, four with BWS and five with IHH. Among BWS patients, all were males, while in the IHH group, two were males and three were females. Consanguinity was noted in 50.0% of BWS cases and 20.0% of IHH patients. There was no family history of BWS or tumors in either group. The most common cardinal feature of clinically diagnosed BWS patients was macroglossia (4/4), followed by hepatosplenomegaly (3/4), omphalocele (2/4), and lateralized overgrowth (1/4). Among major suggestive features, hernia, bilateral kidney enlargement, hypoglycemia, and nephrological abnormalities were identified in 2/4 BWS patients, followed by macrocephaly, microcephaly, and Mongolian spots (1/4). In IHH patients, lateralized overgrowth was a constant feature followed by macrocephaly and unilateral kidney enlargement (2/5). Other features, including hernia, hepatosplenomegaly, neurological, nephrological abnormalities, cardiac defects, and Mongolian spots, were less common (1/5). No tumors were detected in either group (0/9).
Table 1: Demographic, clinical, and molecular findings of Beckwith-Wiedemann syndrome (BWS) and isolated hemihyperplasia (IHH) patients.
|
Gender
|
|
|
|
Males
|
4
|
2
|
|
Female
|
0
|
3
|
|
Consanguinity
|
2
|
1
|
|
Family history of BWS
|
0
|
0
|
|
Family history of tumor
|
0
|
0
|
|
Clinical features
|
|
|
|
Macroglossia
|
4
|
0
|
|
Omphalocele
|
2
|
0
|
|
Hernia
|
2
|
1
|
|
Lateralized overgrowth
|
1
|
5
|
|
Tumor (e.g, Wilms,
hepatoblastoma)
|
0
|
0
|
|
Hyperinsulinism
|
0
|
0
|
|
Organomegaly
|
4
|
3
|
|
Hepatomegaly
|
3
|
1
|
|
Enlarged kidney
|
2 (bilateral)
|
2 (unilateral)
|
|
Hypoglycemia
|
2
|
0
|
|
Neurological abnormalities
|
0
|
1
|
|
Nephrological abnormalities
|
2
|
1
|
|
Cardiac defects
|
0
|
1
|
|
Macrocephaly
|
1
|
2
|
|
Microcephaly
|
1
|
0
|
BWS: Beckwith-Wiedemann syndrome; IHH: isolated hemihyperplasia.
Table 1 summarizes the clinical and molecular findings of BWS and IHH patients. Further detailed clinical and molecular data are shown in Appendix 1.
All IHH patients were negative for methylation test. BWS patients were all positive for the methylation test, two had LOM at IC2 (22.2%), one had pUPD11 (11.1%), and one had GOM at IC1 (11.1%).
US results showed enlargement of the spleen and liver (hepatosplenomegaly) in 44.4% of the study group, 75.0% of BWS patients, and 20.0% of IHH patients. Bilateral kidney enlargement was noticed by abdominal US in two BWS patients, one of them showed additional tiny echogenic foci that was further studied by MRI and identified as nephrocalcinosis. The US results also showed that 50.0% of BWS patients had bilateral kidney enlargement and 40.0% of IHH patients had unilateral kidney enlargement.
AFP measurements were normal at baseline and in serial follow-up tests for all studied patients except one patient who had a transient elevation of AFP in early infancy that declined with consequent follow up. Appendix 1 shows the anthropometricand AFP measurements and relevant radiology findings in all BWS and IHH patients included in this study.
Fisher’s exact test was conducted to assess the substantial association between various clinical features and IHH or BWS. It is clearly shown that there is a significant association between macroglossia and organomegaly with BWS (p = 0.008 and p = 0.048, respectively). There is also a significant association between lateralized overgrowth and IHH (p = 0.048). Otherwise, no other significant association was noted.
Discussion
This study conducted a retrospective cohort analysis to examine the clinical features and molecular abnormalities of patients diagnosed with BWSp who attended the genetic clinic at SQUH over the past decade.
Macroglossia (100%) and hepatomegaly (75.0%) were the most common feature in our BWS cohort. Other features such as hernia, bilateral kidney enlargement, hypoglycemia at birth, nephrological abnormalities, and omphalocele, were evident in 50.0% of cases. Furthermore, lateralized overgrowth, macrocephaly microcephaly, and Mongolian spots were noted in 25.0% of cases. Our findings align with a previous study, revealing that among clinical symptoms, macroglossia was the most prevalent, observed in up to 97% of patients. Other features such as omphalocele and umbilical hernia were present in 80% of BWS patients.13 Likewise, macroglossia was the most common clinical features in clinically diagnosed BWS patients (71.4%) followed by umbilical hernia (65.0%) in another study.10 Additionally, a retrospective study conducted across multiple tertiary centers in Hong Kong found that macroglossia (70.4%) and abdominal wall defects (70.4%) were the predominant clinical features.14
In our study, lateralized overgrowth was consistently observed in all IHH patients (100%) and was present in one patient with BWS. This feature has been noted as predominant, occurring in 51.1% of IHH patients in the West Midlands and 64% of BWS patients in previous reports.4,13,15
Given the high risk of tumor in BWS, patients with IC1 GOM and pUPD11 have significantly higher tumor incidences (22.8–28.6% and 13.8–17.3%, respectively) than those with IC2 LOM (2.5–3.1%).3,4,8,9,16,17 Moreover, patients without molecular abnormalities have a tumor prevalence around 6.7%.8,16,17 Additionally, patients with IC1 GOM had a significantly higher incidence of multifocal/bilateral Wilms tumor compared to patients with pUPD11.9 Fortunately, our cohort study did not identify any BWS-related embryonal tumors. This could potentially be attributed to the limited number of patients included in our study. In contrast, another study reported that among 1370 patients diagnosed with BWS, 102 developed tumors (7.4%). Within this group, those with IC1 GOM had the highest tumor prevalence at 22.8%.8 Additionally, Maas et al,18 found an 8% tumor risk among 1971 BWS patients with IC2 LOM showing the highest tumor prevalence at 28%. Similarly, in a retrospective multicenter study conducted in Hong Kong involving 27 molecularly confirmed BWS patients, 7.4% were found to have embryonal tumors, with IC2 LOM being the most prevalent at 41.8%.10,14
Molecular testing using MLPA revealed that all patients diagnosed with BWS had positive methylation test results. IC2 LOM was the most frequent abnormality, occurring in 50.0% of cases, followed by IC1 GOM and pUPD11, each observed in 25.0% of patients. This was consistent with findings from a study involving BWS patients in North America and Europe, where hypomethylation in the BWS critical region (IC2 LOM) was reported in 50% of cases and other molecular abnormalities noted included hypermethylation in the BWS locus (2–7%), pUPD (20%), mutations in CDKN1C (10%), 11p15 duplications (1%), and inversions or translocations involving 11p15 (1%).8,19 On the contrary, in Japanese patients with BWS, no cases were identified with complete hypermethylation, whereas the incidence of chromosomal abnormalities was higher, noted at 13%. These observations indicate that susceptibility to epigenetic and genetic alterations may vary depending on ethnicity.19 All IHH patients were negative for the methylation test. IHH, which can be considered a mosaic form of overgrowth, may be caused by mosaic genetic defects. In particular, pUPD, which is always found in a mosaic form, is associated with enlarged organs that contain a higher proportion of disomic cells. For diagnostic purposes, methylation patterns are typically assessed in blood lymphocytes, not in the hyperplastic tissues themselves. In some unexplained cases of IHH, pUPD may only be present in the hyperplastic tissue and not in the blood, and therefore missed by routine diagnostic testing.20,21
Several strategies for tumor surveillance in BWSp have been proposed, often involving abdominal US with or without AFP testing at various ages and intervals during infancy.3 In our study group, AFP levels were generally within normal limits, except for one BWS patient who exhibited transiently elevated AFP levels in early infancy that subsequently decreased during follow up visits. Serial abdominal US examinations in our cohort did not identify any tumors.
Previous studies found that patients with IC2 LOM had greater rates of omphalocele, macroglossia, ear creases/pits, face nevus simplex, and preterm and lower rates of organomegaly, lateralized overgrowth, and malignancies.9,20 Our study showed similar findings where patients with LOM had macroglossia, transient hypoglycemia at birth, mild hepatosplenomegaly by US, hernia (umbilical and inguinal), and prematurity. Other reports found that patients with pUPD11 had a considerably higher rate of lateralized overgrowth.9,20 The only patient among our cohort with pUPD had lateralized overgrowth with body asymmetry and bilateral kidney enlargement.
Based on statistical analysis conducted within our patient cohort, we observed significant associations between macroglossia and organomegaly with BWS. Conversely, lateralized overgrowth was significantly associated with IHH, while no significant differences were noted in other features between BWS and IHH patients.
Conclusion
This study represents the first characterization of clinical and molecular abnormalities in Omani patients with BWsp. Macroglossia was the predominant feature in BWS patients, while lateralized overgrowth was universally present in IHH patients. Methylation abnormalities were exclusive to BWS patients, with IC2 LOM as the most common. No tumors were detected in this cohort. Further multicenter studies are recommended to deepen understanding of genotype-phenotype correlations, tumor risks, and to compare findings across diverse populations.
Disclosure
The authors declare no conflicts of interest. No funding was received for this study.
Acknowledgments
We would like to thank the patients and their families for their contribution with their information in our research. Also, many thanks to Sultan Qaboos University Hospital and the Department of Genetics with their physicians and staff.
references
- 1. Mussa A, Russo S, De Crescenzo A, Chiesa N, Molinatto C, Selicorni A, et al. Prevalence of Beckwith-Wiedemann syndrome in North West of Italy. Am J Med Genet A 2013 Oct;161A(10):2481-2486.
- 2. Eggermann T, Perez de Nanclares G, Maher ER, Temple IK, Tümer Z, Monk D, et al. Imprinting disorders: a group of congenital disorders with overlapping patterns of molecular changes affecting imprinted loci. Clin Epigenetics 2015 Nov;7(1):123.
- 3. Brioude F, Kalish JM, Mussa A, Foster AC, Bliek J, Ferrero GB, et al. Expert consensus document: clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: an international consensus statement. Nat Rev Endocrinol 2018 Apr;14(4):229-249.
- 4. Weksberg R, Shuman C, Beckwith JB. Beckwith-Wiedemann syndrome. Eur J Hum Genet 2010 Jan;18(1):8-14.
- 5. Choufani S, Shuman C, Weksberg R. Beckwith-Wiedemann syndrome. Am J Med Genet C Semin Med Genet 2010 Aug;154C(3):343-354.
- 6. Eggermann T, Algar E, Lapunzina P, Mackay D, Maher ER, Mannens M, et al. Clinical utility gene card for: Beckwith-Wiedemann syndrome. Eur J Hum Genet 2014 Mar;22(3):435-435.
- 7. Weksberg R, Shuman C, Smith AC. Beckwith-Wiedemann syndrome. Am J Med Genet C Semin Med Genet 2005 Aug;137C(1):12-23.
- 8. Mussa A, Molinatto C, Baldassarre G, Riberi E, Russo S, Larizza L, et al. Cancer risk in Beckwith-Wiedemann syndrome: a systematic review and meta-analysis outlining a novel (Epi)genotype specific histotype targeted screening protocol. J Pediatr 2016 Sep;176:142-149.e1.
- 9. Duffy KA, Grand KL, Zelley K, Kalish JM. Tumor screening in Beckwith-Wiedemann syndrome: parental perspectives. J Genet Couns 2018 Aug;27(4):844-853.
- 10. Wang R, Xiao Y, Li D, Hu H, Li X, Ge T, et al. Clinical and molecular features of children with Beckwith-Wiedemann syndrome in China: a single-center retrospective cohort study. Ital J Pediatr 2020 Apr;46(1):55.
- 11. Calvello M, Tabano S, Colapietro P, Maitz S, Pansa A, Augello C, et al. Quantitative DNA methylation analysis improves epigenotype-phenotype correlations in Beckwith-Wiedemann syndrome. Epigenetics 2013 Oct;8(10):1053-1060.
- 12. Mussa A, Russo S, Larizza L, Riccio A, Ferrero GB. (Epi)genotype-phenotype correlations in Beckwith-Wiedemann syndrome: a paradigm for genomic medicine. Clin Genet 2016 Apr;89(4):403-415.
- 13. Fontana L, Tabano S, Maitz S, Colapietro P, Garzia E, Gerli AG, et al. Clinical and molecular diagnosis of Beckwith-Wiedemann syndrome with single- or multi-locus imprinting disturbance. Int J Mol Sci 2021 Mar;22(7):3445.
- 14. Luk HM. Clinical and molecular characterization of Beckwith-Wiedemann syndrome in a Chinese population. J Pediatr Endocrinol Metab 2017 Jan;30(1):89-95.
- 15. Radley JA, Connolly M, Sabir A, Kanani F, Carley H, Jones RL, et al. Isolated- and Beckwith-Wiedemann syndrome related-lateralised overgrowth (hemihypertrophy): clinical and molecular correlations in 94 individuals. Clin Genet 2021 Sep;100(3):292-297.
- 16. Brioude F, Lacoste A, Netchine I, Vazquez MP, Auber F, Audry G, et al. Beckwith-Wiedemann syndrome: growth pattern and tumor risk according to molecular mechanism, and guidelines for tumor surveillance. Horm Res Paediatr 2013;80(6):457-465.
- 17. Mussa A, Di Candia S, Russo S, Catania S, De Pellegrin M, Di Luzio L, et al. Recommendations of the scientific committee of the Italian Beckwith-Wiedemann syndrome association on the diagnosis, management and follow-up of the syndrome. Eur J Med Genet 2016 Jan;59(1):52-64.
- 18. Maas SM, Vansenne F, Kadouch DJ, Ibrahim A, Bliek J, Hopman S, et al. Phenotype, cancer risk, and surveillance in Beckwith-Wiedemann syndrome depending on molecular genetic subgroups. Am J Med Genet A 2016 Sep;170(9):2248-2260.
- 19. Sasaki K, Soejima H, Higashimoto K, Yatsuki H, Ohashi H, Yakabe S, et al. Japanese and North American/European patients with Beckwith-Wiedemann syndrome have different frequencies of some epigenetic and genetic alterations. Eur J Hum Genet 2007 Dec;15(12):1205-1210.
- 20. Bliek J, Maas S, Alders M, Merks JH, Mannens M. Epigenotype, phenotype, and tumors in patients with isolated hemihyperplasia. J Pediatr 2008 Jul;153(1):95-100.
- 21. Mussa A, Carli D, Cardaropoli S, Ferrero GB, Resta N. Lateralized and segmental overgrowth in children. Cancers (Basel) 2021 Dec;13(24):6166.