Carcinoid tumors are rare, well-differentiated malignancies that arise from enterochromaffin neuroendocrine cells found across the gastrointestinal (GI) tract. They constitute < 1% of all visceral malignancies and may metastasize to the liver, leading to carcinoid syndrome.1 Immunologically, carcinoid tumor cells evade T-lymphocyte-mediated destruction by expressing large amounts of PD-L 1, promoting carcinoid survival.2 Despite their malignant nature, these tumors are usually slow-growing and discovered incidentally during endoscopy. When symptomatic, they commonly cause vague abdominal pain.3 In about 10% of cases, carcinoid syndrome occurs as the tumor metastasizes to the liver, leading to the release of excessive serotonin into the bloodstream.4 In the GI tract, carcinoid tumors most commonly arise from the small bowel (45%), less commonly from the colon (11%), or stomach (8%).5 Duodenal carcinoid tumors account for < 1% of all GI carcinoids and rarely bleed or cause iron deficiency anemia.6 Here, we report an unusual case of a duodenal carcinoid tumor presenting as iron deficiency anemia.
Case Report
A 71-year-old male was referred to the gastroenterology outpatient clinic by his primary care physician due to new-onset anemia. His hemoglobin upon presentation was 7.5 gm/dL, a drop from 11.7 gm/dL two months before presentation. Complete blood count showed a mean corpuscular volume of 75 fL, white blood cell count of 6.1 x 109/L, and platelet count of 147 x 109/L. His basic metabolic profile showed normal electrolytes, kidney function, and liver function tests. His medical history included atrial fibrillation with home rivaroxaban, chronic obstructive pulmonary disease, congestive heart failure, diabetes mellitus, and reflux disease. One year before presentation, he had intermittent rectal bleeding, which was investigated by colonoscopy, revealing internal bleeding hemorrhoids that were successfully banded. On presentation, the patient reported postural dizziness, weakness, and fatigue for several weeks before seeking medical attention, prompted by his primary care provider’s order for a hemoglobin level test. He also reported black stools, which he attributed to his oral iron supplements. He reported no blood per rectum, hematemesis, or coffee-ground emesis. Additionally, no rash, shortness of breath, or palpitations were reported. The review of systems was otherwise negative. The patient’s vital signs were within normal range upon presentation. Physical examination revealed a thin and frail African-American man with clear lung sounds on auscultation and no abdominal tenderness or masses. Other aspects of the physical examination were unremarkable.
We planned to perform an upper endoscopy and colonoscopy to investigate the source of the GI bleeding, given the patient’s anticoagulation use and previous history of rectal bleeding. A colonoscopy was initially performed, which showed a 5 mm tubular adenoma near the hepatic flexure, deemed unlikely to cause his anemia. Subsequently, an upper endoscopy was then performed, revealing non-bleeding gastric erosions with benign features in the gastric antrum. Advancing the scope to the duodenum uncovered a single 12 mm ulcerated bleeding nodule in the second portion of the duodenum, proximal to the major papilla [Figure 1].
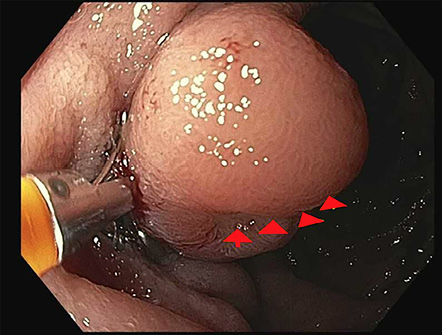 Figure 1: A single 12 mm ulcerated bleeding nodule in the second portion of the duodenum just proximal to the major papilla.
Figure 1: A single 12 mm ulcerated bleeding nodule in the second portion of the duodenum just proximal to the major papilla.
Biopsies of the duodenal nodule were taken with cold forceps and sent for pathology. Pathological analysis of the biopsied nodule revealed a 1.5 cm well-differentiated neuroendocrine tumor with carcinoid features extending to the submucosa layer. To further classify the tumor, immunohistochemistry staining was performed. The neoplastic cells stained positive for CK8/18, CD56, and synaptophysin and were focally positive for chromogranin. This staining pattern confirmed the diagnosis of a duodenal carcinoid tumor.
The patient was then evaluated in the oncology outpatient clinic, and the workup ordered included an iron panel, positron emission tomography scan, 24-hour urinary 5-hydroxyindoleacetic acid, and chromogranin level to evaluate the extent and possible metastasis of the lesion. The positron emission tomography scan revealed mild heterogeneous tracer localization involving the duodenum and no evidence of metastasis. Iron studies showed iron levels of 21 mcg/dL, ferritin of 31 ng/dL, and transferrin saturation of 5.6%, confirming iron deficiency anemia. The 24-hour urine 5-hydroxyindoleacetic acid was 1.1 mg/L, and the serum chromogranin A level was 1725 ng/mL, confirming the absence of metastasis.
Due to significant comorbidities and extensive medical history, the patient was deemed unfit for surgery and was referred back to the gastroenterology clinic for endoscopic resection of the tumor. The patient was planned for endoscopic mucosal resection of the tumor, and an upper endoscopy was performed. The scope advancement revealed a single 12 mm centrally ulcerated submucosal nodule away from the ampulla in the second portion of the duodenum. The area was successfully injected with 7–8 mm saline for lesion assessment, and the injection appeared to lift the lesion adequately. A 12–15 mm area was resected, and retrieval was complete [Figure 2].
 Figure 2: A 12 mm nodule was resected and retrieval was complete.
Figure 2: A 12 mm nodule was resected and retrieval was complete.
There was no bleeding throughout the procedure. The patient was then instructed to follow up with the gastroenterology clinic for endoscopic tumor surveillance. Informed consent was obtained from the patient. Unfortunately, the patient was admitted to the hospital multiple times for other medical reasons and passed away before surveillance and follow-up could be completed.
Discussion
Carcinoid tumors are rare neoplasms that originate from a wide range of organ systems. In the GI tract, they account for < 2% of all GI neoplasms.1 Duodenal carcinoids are the rarest among GI carcinoid tumors, representing only 2.6% of all GI carcinoid tumors in the USA.6 Due to their rarity, there are limited studies in the literature assessing duodenal carcinoids, with most articles including only a small number of patients with this diagnosis.7
Waisberg et al,8 reviewed the clinical characteristics of 24 patients with a pathological diagnosis of duodenal carcinoids at their institution. The most common clinical symptoms reported in their study were dyspepsia (50%), epigastric pain (45%), and, less commonly, weight loss and vomiting.8 Notably, none of the patients exhibited symptoms or laboratory values indicative of iron deficiency anemia. Furthermore, our extensive literature review found no reported cases of duodenal carcinoids causing iron deficiency anemia, as observed in our patient.
Dutta and Panda9 reported an interesting case of an ileal carcinoid tumor causing severe lower GI bleeding and an abrupt decrease in baseline hemoglobin levels, resulting in hemodynamic instability. The patient had undergone an esophagogastroduodenoscopy (EGD) and colonoscopy six months prior to presentation, with no detection of a tumor. Similar to our case, the suspicion of a carcinoid tumor causing GI bleeding was initially low. Ultimately, an emergent laparotomy was required to address the bleeding caused by the ileal carcinoid.9 Fortunately, in our case, the tumor was located in the upper half of the duodenum, making it easily detectable by EGD. In the upper GI tract, Thongtan et al,10 reported a similar case involving a gastric carcinoid tumor presenting with melena of one-day duration and hypotension. An EGD detected the gastric carcinoid, which subsequently metastasized to the liver and led to the patient’s death within two months.10 Although exceedingly rare, there have been reports of pancreatic carcinoids causing GI bleeding by either metastasizing to the stomach and inducing ulcers, or by infiltrating the portal venous system, resulting in variceal formation and subsequent GI bleeding.11
Most duodenal carcinoid tumors reported in the literature were < 2 cm in size and were discovered through upper endoscopy.12 While limited data are available to guide the choice of intervention for such tumors, carcinoid tumors, in general, have a propensity for lymph node involvement. Surgical intervention was the predominant treatment for most cases of duodenal carcinoids in the literature.13,14 Across the literature, duodenal carcinoids with a diameter of < 10 mm were successfully managed by endoscopic mucosal resection alone, with minimal risk of recurrence. However, for tumors > 10 mm but < 2 cm, the limited data across the literature has yielded conflicting recommendations between local excision and more aggressive surgical approaches.15 In our patient’s case, with a tumor diameter of 12 mm, endoscopic resection was the only viable treatment option given that the patient was highly unfit for surgery with a high risk of mortality if surgery was attempted. Nevertheless, additional data and reports are needed to establish a more robust evidence base for the management of duodenal carcinoid tumors.
Conclusion
This case report highlights the unusual occurrence of a duodenal carcinoid tumor causing iron deficiency anemia secondary to slow GI bleeding. While carcinoid tumors rarely contribute to GI bleeding, they should be considered in the differential diagnosis when evaluating a patient with iron deficiency anemia and slow GI bleeding. Furthermore, clinicians should be encouraged to publish all cases of duodenal carcinoids presenting with GI bleeding to increase the number of reported cases in the literature, which may facilitate further research on the best approaches to managing similar diagnoses.
Disclosure
The authors declare no conflicts of interest.
references
- 1. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008 Jun;26(18):3063-3072.
- 2. Cives M, Strosberg J, Al Diffalha S, Coppola D. Analysis of the immune landscape of small bowel neuroendocrine tumors. Endocr Relat Cancer 2019 Jan;26(1):119-130.
- 3. Robertson RG, Geiger WJ, Davis NB. Carcinoid tumors. Am Fam Physician 2006 Aug;74(3):429-434.
- 4. Pinchot SN, Holen K, Sippel RS, Chen H. Carcinoid tumors. Oncologist 2008 Dec;13(12):1255-1269.
- 5. Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med 1999 Mar;340(11):858-868.
- 6. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003 Feb;97(4):934-959.
- 7. Mullen JT, Wang H, Yao JC, Lee JH, Perrier ND, Pisters PW, et al. Carcinoid tumors of the duodenum. Surgery 2005 Dec;138(6):971-977; discussion 977-978.
- 8. Waisberg J, Joppert-Netto G, Vasconcellos C, Sartini GH, Miranda LS, Franco MI. Carcinoid tumor of the duodenum: a rare tumor at an unusual site. Case series from a single institution. Arq Gastroenterol 2013;50(1):3-9.
- 9. Dutta G, Panda M. An uncommon cause of lower gastrointestinal bleeding: a case report. Cases J 2008 Oct;1(1):235.
- 10. Thongtan T, Deb A, Islam S, Nugent K. Upper gastrointestinal bleeding as the initial manifestation of gastroenteropancreatic neuroendocrine tumors. Proc (Bayl Univ Med Cent) 2021 Apr;34(5):618-619.
- 11. Zafar Y, Doran S, Helzberg J. Metastatic neuroendocrine tumor of the pancreas presenting with gastric variceal hemorrhage. Off J Am Coll Gastroenterol 2018;113:S1143-S1144.
- 12. Cheek RC, Wilson H. Carcinoid tumors. Curr Probl Surg 1970 Nov;4-31.
- 13. Burke AP, Sobin LH, Federspiel BH, Shekitka KM, Helwig EB. Carcinoid tumors of the duodenum. A clinicopathologic study of 99 cases. Arch Pathol Lab Med 1990 Jul;114(7):700-704.
- 14. Zyromski NJ, Kendrick ML, Nagorney DM, Grant CS, Donohue JH, Farnell MB, et al. Duodenal carcinoid tumors: how aggressive should we be? J Gastrointest Surg 2001;5(6):588-593.
- 15. Dalenbäck J, Havel G. Local endoscopic removal of duodenal carcinoid tumors. Endoscopy 2004 Jul;36(7):651-655.