Primary squamous cell carcinoma (SCC) of the renal parenchyma represents less than 0.5% of all renal tumors. It is usually associated with squamous metaplasia due to chronic irritation, a history of kidney stones, and/or recurrent urinary tract infections.1 Only a few case reports exist about renal SCC without the abovementioned risk factors. The delay in diagnosis following deceptive symptomatology, without specific signs, often leads to a pejorative evolution, leading to late-stage discovery and poor prognosis.2 Here, we report three rare cases with clinical presentation, etiology, and outcomes. Verbal consent was taken from the patient.
Case Reports
Case one
A 67-year-old diabetic man presented to the urology clinic with painless gross hematuria associated with clots. He denied any history of urolithiasis or recurrent urinary tract infections. Examination revealed a ballotable mass on the right side of the abdomen. Urine cytology was negative for malignant urothelial cells. Computed tomography (CT) of the chest and abdomen demonstrated a right renal mass with no metastasis, suspecting renal cell carcinoma (RCC) [Figure 1]. The patient underwent a right laparoscopic radical nephrectomy, and the postoperative course was uneventful. A repeat CT scan after the surgery found no early recurrence or residual disease.
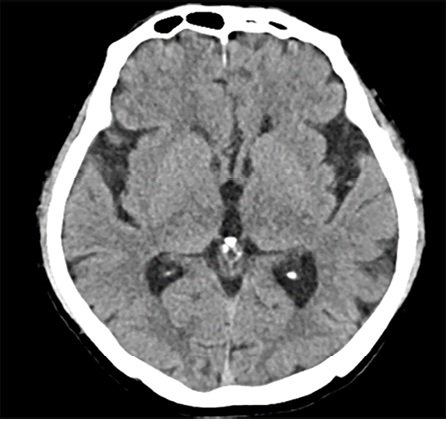 Figure 1: (Case 1) CT scan of abdomen and pelvis.
Figure 1: (Case 1) CT scan of abdomen and pelvis.
Histopathology showed a moderately-to-poorly differentiated 8 × 6 cm squamous cell carcinoma arising from the right kidney. The tumor cells showed a diffuse pattern and exhibited strong positivity for cytokeratin (CK) markers CK14 and CK5/6, in addition to the p63 marker. The tumor cells were negative for GATA3, uroplakin, RCC, and PAX8, and the associated squamous metaplastic lining of the renal pelvicalyceal system exhibited dysplasia highlighted by strong positivity for CK5/6, p63, and CK14, and negativity for uroplakin and GATA3 [Figures 2–4]. the pathological stage was pT4Nx. CT scans were performed at the last follow-up, nine months after surgery, and showed no evidence of disease recurrence. Cystoscopy did not show any tumor in the bladder, and the barbotage to the right ureter sent for cytology was negative for malignant cells. During the patient's last visit to the urology clinic, he was in good health and asymptomatic. A CT scan is scheduled for his next follow-up.
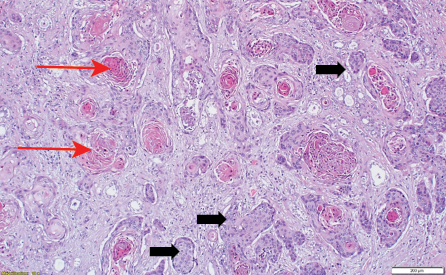 Figure 2: (Case 1) Photomicrograph showing nests of pleomorphic tumor cells (red arrows) together with keratin pearl formation (black arrows) hematoxylin and eosin stain, magnification = 200 ×.
Figure 2: (Case 1) Photomicrograph showing nests of pleomorphic tumor cells (red arrows) together with keratin pearl formation (black arrows) hematoxylin and eosin stain, magnification = 200 ×.
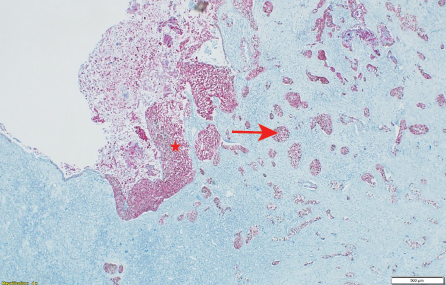 Figure 3: (Case 1) Photomicrograph showing p63 and CK5/6 immunohistochemical stains in dysplastic squamous mucosa (star) and in the invasive tumor (arrow) hematoxylin and eosin stain, magnification = 500 ×.
Figure 3: (Case 1) Photomicrograph showing p63 and CK5/6 immunohistochemical stains in dysplastic squamous mucosa (star) and in the invasive tumor (arrow) hematoxylin and eosin stain, magnification = 500 ×.
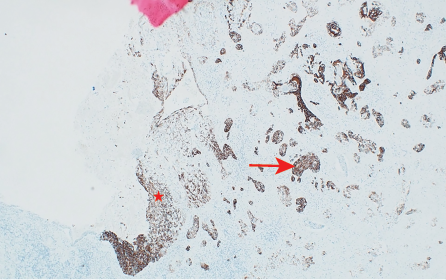 Figure 4: (Case 1) Photomicrograph showing CK14 immunohistochemical stain positive in dysplastic mucosa (star) and in the invasive carcinoma (arrow) hematoxylin and eosin stain, magnification = 100 ×.
Figure 4: (Case 1) Photomicrograph showing CK14 immunohistochemical stain positive in dysplastic mucosa (star) and in the invasive carcinoma (arrow) hematoxylin and eosin stain, magnification = 100 ×.
Case two
A 50-year-old woman was referred to the urology clinic with a tumor in the right renal and a nephrocutaneous fistula. She had been complaining of right-sided flank pain for four months. She underwent surgery for a psoas muscle abscess more than 30 years ago. On physical examination, a fistulous opening was seen in the right flank area with minimal discharge.
A CT scan showed a large tumor occupying the whole right kidney [Figure 5]. Magnetic resonance imaging of the abdomen revealed a large heterogeneous lesion occupying the right kidney, highly suggestive of a neoplastic etiology. Multiple enlarged retroperitoneal lymph nodes were also visualized. There was a loss of fat plane between the right colon and the mass [Figures 6 and 7]. The patient underwent open right radical nephrectomy, along with an excision of the nephrocutaneous fistula tract. Due to adhesions with the right colon and suspected local invasion, enbloc resection with right hemicolectomy was also performed.
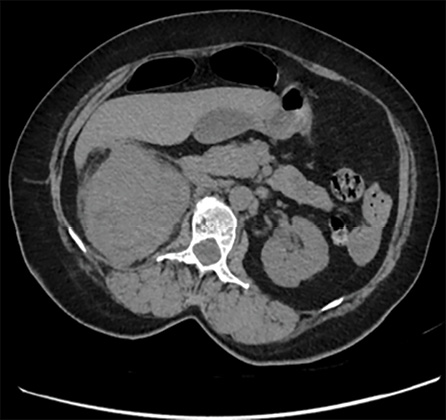 Figure 5: (Case 2) CT scan of the abdomen and pelvis showing a large mass in the right renal mass area.
Figure 5: (Case 2) CT scan of the abdomen and pelvis showing a large mass in the right renal mass area.
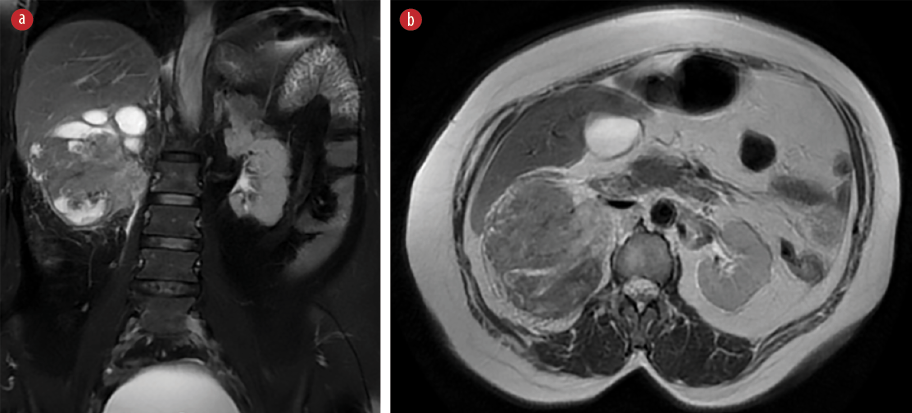 Figure 6: (Case 2) Magnetic resonance imaging of abdomen showing a large heterogeneous lesion occupying the right kidney, highly suggestive of a neoplastic etiology. (a) Coronal view (b) Axial cut section
Figure 6: (Case 2) Magnetic resonance imaging of abdomen showing a large heterogeneous lesion occupying the right kidney, highly suggestive of a neoplastic etiology. (a) Coronal view (b) Axial cut section
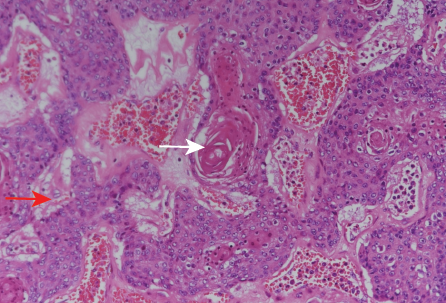 Figure 7: (Case 2) Photomicrograph of the tumor showing nests of pleomorphic tumor cells (red arrow) together with keratin pearl formation (white arrow), hematoxylin and eosin stain, magnification = 200 ×.
Figure 7: (Case 2) Photomicrograph of the tumor showing nests of pleomorphic tumor cells (red arrow) together with keratin pearl formation (white arrow), hematoxylin and eosin stain, magnification = 200 ×.
Histopathology revealed moderately differentiated squamous cell carcinoma arising from the pelvicalyceal system with a tumor size of 10 cm. The tumor extended into the perinephric soft tissue and obliterated the renal sinus. The tumor infiltrated the pericolonic fat and nephrocutaneous fistula, and lymphovascular invasion was present.
All margins and regional lymph nodes were negative. [Figure 7].
Case three
A 45-year-old man, who was a smoker for 20 years, presented to the urology clinic with a month-long history of left flank pain and dysuria. He denied any history of hematuria.
CT scan of the abdomen (sent by the referring physician) showed a large complex cystic mass with calcification and solid components. Multiple para-aortic lymph nodes and a single 1 cm liver lesion were seen. The patient underwent a left radical nephrectomy. The histopathology report showed a well-differentiated SCC of the kidney with metastatic deposits on the lymph nodes. [Figure 8].
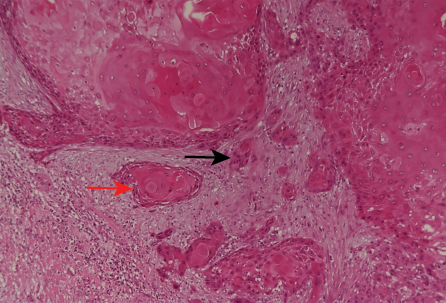 Figure 8: (Case 3) Photomicrograph of tumor showing nests of pleomorphic tumor cells (black arrow) together with keratin pearl formation (red arrow), hematoxylin and eosin stain, magnification = 200 ×.
Figure 8: (Case 3) Photomicrograph of tumor showing nests of pleomorphic tumor cells (black arrow) together with keratin pearl formation (red arrow), hematoxylin and eosin stain, magnification = 200 ×.
Postoperatively, six cycles of adjuvant chemotherapy with 5-fluorouracil and cisplatin were planned but had to be modified after the first cycle due to significant toxicities, including gastrointestinal grade I toxicity and febrile neutropenia requiring IV antibiotics (grade III). After three cycles of reduced-dose chemotherapy, a CT scan showed some reduction in the disease. After six cycles post-treatmen, positron emission tomography-CT showed fludeoxyglucose avid nodular lesions in the left renal fossa, mediastinal lymph nodes, and multiple nodular pulmonary parenchymal lesions representing local and distant recurrence. The patient was prescribed second-line chemotherapy, which he unfortunately could not endure, and he passed away one year following his diagnosis.
Discussion
Primary SCC represents < 4% of epithelial cancers of the upper tract. The risk factors are urolithiasis, recurrent upper urinary tract infections, and urinary schistosomiasis.3 None of our three patients had a history of recurrent/neglected stone or chronic pyelonephritis or schistosomiasis. The absence of specific signs masqueraded by symptomatology of urolithiasis and the infective process may lead to delayed diagnosis. We propose that primary SCC of the kidney may not always be related to a history of recurrent urinary tract infections or renal stone disease but can arise de novo and is associated with poor prognosis.
In our case series, there were different modes of clinical presentation. Case 1 presented with episodes of painless gross hematuria with clots associated with lower urinary tract symptoms. On workup, we did not find any renal stones. Case 2 presented with a nephrocutaneous fistula, and a contrast-enhanced CT scan revealed the presence of a renal mass. In Case 3, there was a long-term history of smoking, flank pain, and dysuria for one month with no recent weight loss.
Transitional cell carcinoma is the most common type of upper tract cancer, originating in the renal pelvis, followed by SCC, predominantly affecting women aged 50–70 years. The age range of our patients was 45–67 years. The most common symptoms were lumbar pain and hematuria. The pain in advanced SCC is attributed to urinary obstruction and/or local extension.3
Our literature review showed a recently published case report of a 79-year-old woman with no history of renal stone disease, recurrent urinary tract infection, or urinary schistosomiasis. She was diagnosed with a renal tumor and underwent nephrectomy.4 Histopathology revealed primary invasive SCC of renal parenchyma associated with foci of carcinoma in situ on squamous metaplasia in the calyxes. Unlike many published cases of primary SCC of the kidney, our patients did not report any history of renal stones, urinary tract infections, or urinary schistosomiasis.
The histopathological features of SCC on the cut section classically show renal parenchyma with an irregular surface. Heterogeneous tumors with solid and cystic components occupy a large portion of the kidney and may be associated with dual pathology. The tumor usually occupies most of the kidney and extends into perirenal adipose tissue, as seen in our cases. Microscopic examination demonstrates renal parenchyma with areas of metaplastic stratified squamous epithelium and dysplastic squamous mucosa. Such tumors are likely to be invasive, moderately to poorly differentiated, and mostly arranged in clusters and nests. Immunohistochemistry of renal SCC demonstrates the presence of p63 along with CK5/6 stains in the dysplastic squamous epithelium or the invasive tumor without GATA-3 and the uroplakin staining in the mucosa and aggressive tumor.2 All these microscopic and immunohistochemical features were found in our patients. Fotovat et al,5 reported such clinicopathological structures in a case of aggressive primary renal SCC. Significant histological findings of SCC are keratin pearl formation and intercellular bridges; renal rubber consistency and pasty keratin secretions were important findings in all three of our patients.5
Ghosh and Saha6 reported a case similar to ours in a 51-year-old man who presented with a complaint of right flank pain, not associated with stone disease. On contrast-enhanced CT scan, a right renal lesion was detected, and the patient underwent a total nephrectomy. Histopathology reported a well-differentiated SCC with nests of large atypical squamous epithelial cells, associated with other features like keratin pearl formation, and focal area of necrosis in intra-renal parenchyma with entrapped glomeruli and tubules. The pelvicalyceal system was free from tumors. No recurrence was found after 12 months of follow-up.6
Adjuvant or neoadjuvant chemotherapy is often used in metastatic SCC of the renal pelvis with a combination of cisplatin, methotrexate, and bleomycin, though with limited patient survival benefits.5 Studies evaluating the role of chemotherapy in primary SCC of renal parenchyma have shown poor outcomes with a lack of an efficient treatment regime. Our patient (case 3) received six cycles of 5-fluorouracil and cisplatin with overall poor tolerability and a lack of response to treatment, which eventually led to his death. In our experience, surgery at an early stage may be the best curative treatment. However, people with SCC of the renal pelvis usually present at advanced stages, diminishing the odds of their survival.7
Conclusion
Primary renal SCC is a rare and aggressive disease whose symptoms may not be apparent till very late, leading to delayed diagnosis and poor outcome. However, early detection significantly enhances favorable results through radical nephrectomy. Early intervention is key to improving patient prognosis.
Disclosure
The authors declare no conflict of interest.
references
- 1. Kose F, Bal N, Ozyilkan O. Squamous cell carcinoma of the renal pelvis. Med Oncol 2009;26(1):103-104.
- 2. Zhang X, Zhang Y, Ge C, Zhang J, Liang P. Squamous cell carcinoma of the renal parenchyma presenting as hydronephrosis: a case report and review of the recent literature. BMC Urol 2020 Jul;20(1):107.
- 3. Slimane M, Hadidane M, Bouzaiene H, Driss M, Jaidane O, Henchiri H, et al. Squamous cells carcinoma of the renal pelvis discovered due to parietal and skin invasion: an uncommon manifestation. Pan Afr Med J 2018 Dec;31:246.
- 4. Senghor F, Thiam I, Sow O, Traore A, Fall B, Dial CM. Primary squamous cell carcinoma of the kidney in southern Senegal (Ziguinchor): case report and review of the literature. Pan Afr Med J 2021 Nov;40(1):175.
- 5. Fotovat A, Gheitasvand M, Amini E, Ayati M, Nowroozi MR, Sharifi L. Primary squamous cell carcinoma of renal parenchyma: case report and review of literature. Urol Case Rep 2021 Mar; 37:101627.
- 6. Ghosh P, Saha K. Primary intraparenchymal squamous cell carcinoma of the kidney: a rare and unique entity. Case Rep Pathol 2014; 2014:256813.
- 7. Bandyopadhyay R, Biswas S, Nag D, Ghosh AK. Squamous cell carcinoma of the renal pelvis presenting as hydronephrosis. J Cancer Res Ther 2010;6(4):537-539.