Obesity, defined as the abnormal accumulation of fat in the body, is an increasingly prevalent public health issue worldwide.1 According to the World Health Organization,2 adults aged ≥18 years are considered overweight and obese if they have a body mass index (BMI) of 25–29.9 and ≥ 30 kg/m2, respectively. Globally, the prevalence of overweight/obesity has nearly tripled since 1975, with up to 39% and 14% of adults in 2019 considered overweight and obese, respectively.1,3 According to data from the Ministry of Health,4 approximately 60% of adults in Oman were either overweight or obese in 2018.
Obesity can lead to a plethora of chronic and debilitating diseases, including type 2 diabetes mellitus (T2DM), hypertension (HTN), heart disease, stroke, dyslipidemia, and obstructive sleep apnea (OSA), potentially resulting in early mortality.1,5–7 In addition, the treatment of obesity and obesity-related conditions creates a substantial economic burden; for example, obesity alone cost the USA more than USD 1.4 trillion in 2016.8 Hence, finding an effective and sustainable method for weight reduction is important. Compared to nonsurgical interventions, bariatric procedures are reported to be the most effective strategy to induce weight loss.9 In particular, laparoscopic sleeve gastrectomy (LSG) has demonstrated significant efficacy in achieving substantial weight loss and facilitating the remission of most obesity-related comorbidities.10,11
An LSG procedure involves the removal of approximately 70–80% of the greater curvature of the stomach, resulting in the creation of a narrow gastric tube with a volume capacity of ~60–80 mL; thereby, aiding weight loss both by physically restricting food intake as well as by affecting gut hormones to enhance satiety.10,11 Overall, LSG has multiple advantages compared to other types of bariatric surgery, as the procedure results in immediate excess weight loss (EWL), does not interfere with the gastrointestinal anatomy and physiology, avoids dumping syndrome, does not require the insertion of a foreign body at the surgical sites, involves an easy surgical technique, and allows for the possibility of conversion to other bariatric procedures if necessary. On the other hand, there are some disadvantages that should be mentioned, including the irreversibility of the procedure and the possibility of leakage or bleeding at the stapling site.11 The LSG surgery has evolved dramatically over the last two decades, with more than 300 000 procedures conducted annually.11 The global popularity of LSG increased from 4.5% in 2008 to 42.1% in 2013, and as of 2015, it was the most popular bariatric procedure in the USA.11–13
Various studies have investigated the outcomes of LSG with regard to both weight loss and metabolic changes. A study in Kuwait found that LSG resulted in a mean BMI decrease from 47.1 to 34.3 kg/m2 within 5–8 years of surgery.14 A study from the USA reported a percentage EWL of up to 52.8% at six months following LSG in the USA.10 An even
higher percent EWL was recorded in another study, peaking at 81.8% and 78.7% at one and five years follow-up, respectively.15 A study of morbidly obese patients in Egypt,16 concluded that the surgery helped correct metabolic disturbances associated with obesity, such as T2DM, within the immediate postoperative period (i.e., three to six months). Other studies reported a mean percent EWL of 51.1% over a median follow-up period of eight years following LSG,17 and EWL percentages of 76.8%, 69.7%, and 56.1% at one, three, and five years of follow-up, respectively.18 The authors of the study concluded that LSG induced efficient weight loss and a major improvement in obesity-related comorbidities. However, there was a significant rate of weight regain and a decrease in remission rates of T2DM and, to a lesser extent, other comorbidities over time.
Our study aimed to evaluate the effect of LSG on the percent EWL in an obese Omani population at 12 months postoperatively, as well as the effect of the surgery on metabolic parameters and the remission of various comorbidities, including T2DM, HTN, dyslipidemia, and OSA. Additionally, the study attempted to identify patient characteristics influencing remission to determine patient groups most likely to benefit from this procedure. To the best of our knowledge, this is the first retrospective cohort study in Oman seeking to identify the short-term effects of LSG on EWL and metabolic outcomes. This study may pave the way for future research on the long-term effects of LSG and enable researchers to compare outcomes with other types of bariatric surgeries.
Methods
This retrospective cohort study was carried out at the Royal Hospital, a tertiary care institution in Muscat, Oman. This hospital is the main bariatric center in the country to which patients from all over Oman are referred. All obese Omani adult patients aged ≥ 18 years of both genders who underwent LSG between 1 January 2017 and 31 December 2018 were recruited for the study. Patients with BMIs of ≥ 40 kg/m2 scheduled to undergo the surgery were considered eligible for the study, regardless of the presence of any comorbidities. Meanwhile, those with major psychiatric diseases were excluded from the study, as were known drug abusers, female patients who became pregnant in the first year following the surgery, those lost to follow-up during the study period, and those with major postoperative complications.
The necessary sample size was calculated to be 44 using G*Power software (version 3.1.9.2),19 based on Cohen’s d effect size of 0.50 (medium), alpha error of 5%, and at a power of 90%. The adequacy of the sample size was deemed sufficient, considering that approximately 200 LSG procedures are performed annually.
All procedures were performed by the same surgical team and followed the same surgical technique. The surgery was performed by opening five port incisions in different areas of the abdomen before the abdominal cavity was inflated, aiming for the pneumoperitoneum. This technique allowed for clear visualization and assessment of the cavity for any adhesions prior to removal. The first dissection was performed through the gastrocolic ligament approximately 2 cm off the pylorus of the stomach up until the gastroesophageal junction. Subsequently, the stomach was sleeved, starting from 2 cm off the pylorus to 1 cm off the gastroesophageal junction. Thereafter, the remaining portion of the stomach was stapled together. Prior to closure, a leaking test was conducted to check for any oozing or bleeding through the staple line. Finally, the sleeved part of the stomach was removed along with the ports, and small stitches were applied to the site of the incisions.14,16
All patients received the same pre-and post-operative care, including dietary advice and a recommended exercise program. Patients were admitted to the hospital one day before the procedure and discharged on the second day, provided that no major complications occurred. All patients received baseline blood work-ups, including a complete blood count, glycated hemoglobin (HbA1c) measurements, and total lipid profile one to three months preoperatively, with a repeat complete blood count and coagulation profile performed on the day of admission. All patients were discharged on daily proton pump inhibitors and multivitamins. Subsequently, they were followed up as outpatients at three, six, and 12 months postoperatively. At each outpatient visit, changes in weight or improvement in comorbidities were assessed and recorded.
Relevant data were obtained from the patients’ electronic health records. Satisfactory weight loss was set at > 50% EWL for a target BMI of 25 kg/m2.17 Complete remission of T2DM was defined as a normal HbA1c level of < 6.5% without antidiabetic medications, while partial remission was defined as a reduction in the number or dosage of medications necessary.20 Complete remission of HTN was defined as the cessation of antihypertensive medications with normal blood pressure readings (< 140/90 mmHg over multiple readings, if available). Partial remission was defined as a reduction in the number or dosage of medications necessary.21 Complete remission of dyslipidemia was defined as the cessation of lipid-lowering medications with normal laboratory results (total cholesterol < 5.18 mmol/L, high-density lipoprotein [HDL]-cholesterol > 1 mmol/L, low-density lipoprotein [LDL]-cholesterol < 4.1 mmol/L), while partial remission was defined as a reduction in the number or dosage of medications necessary, or cessation of medication use despite abnormal laboratory findings.22 Complete remission of OSA was defined as a major symptomatic relief reported by the patient, without needing further treatment if the patient had previously been on oxygen or continuous positive airway pressure therapy.14 Partial remission was defined as a mild improvement of symptoms with no or only occasional need for treatment using continuous positive airway pressure (i.e., at nighttime or for a few hours in the daytime) or a generally reduced need for treatment compared to before surgery.14
All study variables were analyzed using SPSS Statistics (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.). Continuous variables were expressed as means and SDs, while categorical variables were presented as numbers or percentages. As appropriate, chi-squared tests were used to compare differences in proportion between groups. Cross-tabulation was performed to explore the relationship between two or more categorical variables by recording the frequency distribution. A p-value of < 0.05 was considered statistically significant.
This study received ethical approval from the Research and Ethical Review and Approval Committee of the Centre of Studies and Research at the Ministry of Health, Muscat, Oman (MOH/CSR/20/24120). Informed consent was not necessary because the study was retrospective in nature and did not involve direct intervention or contact with the patients.
Results
A total of 223 patients underwent LSG procedures at the hospital during the study period; 168 (75.3%) fulfilled the inclusion criteria and were included in the study. Overall, 55 patients (24.7%) were excluded as 31 did not receive proper postoperative follow-up care within the first year of surgery, 13 had a BMI of < 40 kg/m2, nine female patients became pregnant within the first year of surgery, one was non-Omani, and one had major bleeding and leakage from the surgical site on the second postoperative day.
Of the 168 patients, the majority were female (n = 111; 66.1%). The mean age at the time of surgery was 36.1 years, and the mean BMI was 50.8 kg/m2. At baseline, 70 patients (41.7%) had obesity-related comorbidities, including T2DM (n = 48; 28.6%), HTN (n = 48; 28.6%), and dyslipidemia (n = 35; 20.8%). A total of 72 patients (42.9%) were diagnosed with OSA [Table 1].
Table 1: Baseline characteristics and prevalence of comorbidities among Omani patients before undergoing a laparoscopic sleeve gastrectomy (N = 168).
|
Age, years
|
34.9 (7.9)
|
36.7 (9.1)
|
36.1 (8.8)
|
|
BMI, kg/m2
|
50.7 (9.3)
|
50.7 (9.3)
|
50.8 (8.8)
|
|
Weight, kg
|
145.4 (28.0)
|
126.0 (21.7)
|
132.6 (25.6)
|
|
T2DM, n (%)
|
16 (28.1)
|
32 (28.8%)
|
48 (28.6)
|
|
HTN, n (%)
|
20 (35.1)
|
28 (25.2%)
|
48 (28.6)
|
|
Dyslipidemia, n (%)
|
13 (22.8)
|
22 (19.8%)
|
35 (20.8)
|
BMI: body mass index; T2DM: type 2 diabetes mellitus; HTN: hypertension; OSA: obstructive sleep apnea.
Data given as mean (SD) unless otherwise indicated.
Most patients achieved significant weight reduction in the first year postoperatively. The mean BMI decreased from 50.8 kg/m2 preoperatively to 34.7 kg/m2 12 months postoperatively. The mean percent EWL was 64.7%, with more than two-thirds of patients (n = 132; 78.6%) achieving the target percent EWL of > 50%. Overall, 51 male patients (89.5%) and 81 female patients (73.0%) achieved the target percent EWL of > 50% (p = 0.014) [Figure 1]. Percent EWL was positively correlated to pre-operative BMI (p < 0.001).
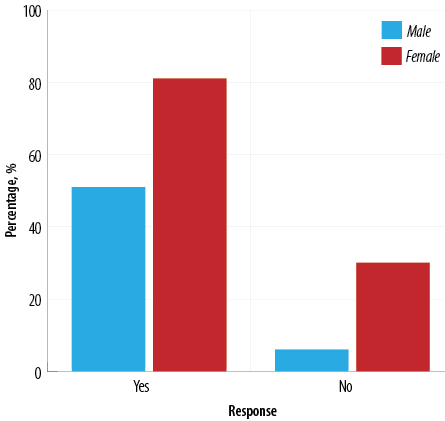 Figure 1: Achievement of target percent excess weight loss (> 50%) 12 months postoperatively among Omani patients undergoing laparoscopic sleeve gastrectomy (N = 168).
Figure 1: Achievement of target percent excess weight loss (> 50%) 12 months postoperatively among Omani patients undergoing laparoscopic sleeve gastrectomy (N = 168).
Of the 48 patients with T2DM at baseline, 31 (64.6%) demonstrated complete remission and 15 (31.3%) partial remission; only one patient (2.1%) showed no change to their medication regimen 12 months postoperatively. Among the 48 patients with HTN, 26 (54.2%) and 16 (33.3%) demonstrated complete and partial remission after LSG, respectively, while three (6.3%) patients had no changes to their medication regimen. With regards to dyslipidemia, 10 (28.6%) of the 35 affected patients were in complete remission at 12 months postoperatively, while two (5.7%) were in partial remission, and 21 (60.0%) experienced no changes to their lipid-lowering medication regimens [Table 2]. At 12 months following the surgery, 29.2% of the 72 patients with preoperative OSA showed improvement in their condition based on their apnea-hypopnea index (AHI) scores; however, a large number of patients had missing data regarding OSA status. Overall, no significant associations were observed between percent EWL and the remission status of any comorbidity (p > 0.050).
Table 2: Remission rates of comorbidities 12 months postoperatively among Omani patients undergoing laparoscopic sleeve gastrectomy (N = 168).
|
T2DM, n (%)
|
31 (64.6)
|
15(31.3)
|
1 (2.1)
|
1 (2.1)
|
|
HTN, n (%)
|
26 (54.2)
|
16 (33.3)
|
3 (6.3)
|
3 (6.3)
|
T2DM: type 2 diabetes mellitus; HTN: hypertension.
Discussion
The mean age and male-to-female ratio for the patients included in our study were comparable to those reported in other relevant studies.14,23 However, the mean preoperative BMI in our study was much higher (50.8 kg/m2) than the average reported in the literature. For instance, Khalaj et al,23 and Kikkas et al,24 reported mean preoperative BMIs of 44.6 and 46.5 kg/m2 among morbidly obese patients undergoing LSG in Iran and Estonia, respectively. Similarly, AlKhaldi et al,14 and Turgut et al,25 reported mean preoperative BMIs of 47.1 and 45.5 kg/m2, respectively, among similar populations in Kuwait and Turkey. With regards to weight loss outcomes, the mean percent EWL for our population at 12 months postoperatively was 64.7%, which is lower than previously reported findings within a similar timeframe (range = 72.8–83.7%).23,25,26 Such variations may be due to the selection of patients with higher BMIs during the initial selection process for bariatric procedures. Our study's higher mean preoperative BMI may explain the lower percent EWL.
In our study, the T2DM remission rate at 12 months following LSG was 64.6%. This falls in line with the outcomes reported in various studies. According to Murshid et al,27 75% of patients undergoing LSG in Saudi Arabia achieved target HbA1c levels of ≤ 6.5% within one year of surgery. Moreover, Lechea et al,28 found that requirements for antidiabetic medical treatment decreased 69% in a Romanian study. Comparably, Al Khayat et al,29 observed a remission rate of 70% among patients with T2DM in Kuwait within the immediate postoperative period (i.e., within one month of LSG). In contrast, Mc Tigue et al,30 found that the adjusted cumulative remission rate for adult patients with T2DM was only 55.9% (95% CI: 53.9–57.9) at 12 months follow-up. This discrepancy in results could be due to study variations in the clinical characteristics of diabetic patients included in the analysis.
Using continuous glucose monitoring, Capoccia et al,31 found that up to 40% of obese T2DM patients who underwent LSG achieved complete remission in hyperglycemia based on an evaluation of glucose variability. The researchers concluded that T2DM duration plays a major role achieving of complete remission of T2DM postoperatively, as patients with a longer duration of T2DM were found to have lower remission rates after bariatric surgery. Similar results were reported by Li et al,32 in a meta-analysis of studies assessing the metabolic effects of bariatric surgery among obese T2DM patients. Based on the analysis of 13 trials involving 357 patients, only 66.35% of patients achieved complete remission and an approximate loss of one-fifth of their body weight when a BMI of < 35 kg/m2 was used as an inclusion criterion. On the other hand, 84% of patients with BMIs of > 35 kg/m2 achieved complete glycemic remission with a loss of almost one-third of their weight.32 Furthermore, 37% of diabetic patients did not receive any medication for diabetes after the surgery and 21% had their doses reduced.
This study demonstrated complete remission of HTN in 54.2% of patients, with 33.3% achieving partial remission. Lechea et al,28 reported that the need for antihypertensive treatment was reduced by 60% post-LSG, while Turgut et al,25 reported a similar remission rate (61%). Another study reported that 52.2% of patients undergoing LSG achieved HTN remission at 12 months.23 According to Kowalewski et al,17 28% of 49 hypertensive patients in Poland did not require pharmacological therapy for HTN after LSG, while 31% had their doses reduced, 33% experienced no changes in treatment, and 8% required increased doses of antihypertensives.
In this study, 28.6% of patients with dyslipidemia at baseline experienced complete remission after 12 months, which is comparable to the rate reported by Khalaj et al,23 among Iranian patients (27.7%). In contrast, Yin et al,33 found that a much higher percentage of 60 morbidly obese patients in China were cured of their dyslipidemia within a year following surgery (86%). Lechea et al,27 noted that requirements for lipid-lowering treatment reduced by 21% following LSG, although 78% of patients still required chronic treatment with statins. The researchers also found that triglycerides and LDL cholesterol decreased by 37% and 9%, respectively, while HDL cholesterol increased by 18% at 12 months following the surgery. Although HDL levels increased significantly, the decline in total cholesterol and LDL was not statistically significant.28 As the continuation of cholesterol-lowering drugs is directed to a greater degree by the patient’s total cholesterol and LDL levels than HDL levels, this may account for the decline in the percentage of complete remission of dyslipidemia in our study. Ruiz-Tovar et al,15 similarly concluded that 21.4% and 100% of patients in Spain achieved complete remission of hypercholesterolemia and hypertriglyceridemia, respectively, 12 months postoperatively. In addition, Hussein34 and Vigilante et al,35 reported substantial improvement in all lipid profile parameters one year after LSG in Egyptian and Italian populations, respectively.
The lack of follow-up data limited our study’s ability to determine the effect of LSG on resolution rates in the long-term postoperative period. A study conducted by Kikkas et al,24 concluded that the remission rate of OSA at five years post-surgery was approximately 61.5%. Further research is recommended to determine whether LSG is effective in resolving OSA in the Omani population. Such studies should utilize the AHI to record the number of apnea- and hypopnea-related events per night during sleep studies to determine whether the surgery resolves or reduces OSA severity.36
This study was subject to several limitations. As the study was conducted at an earlier time, in a single hospital in Oman, and did not include other bariatric centers in the country (e.g., the Armed Forces Hospital or private centers and hospitals), the results of this study might not be generalizable. Moreover, there was a considerable amount of missing data regarding AHI scores; based on the retrospective analysis of the hospital records, a sleep study was not performed for all patients at baseline and, if performed preoperatively, was not repeated postoperatively. Therefore, the OSA findings were based on the patients’ subjective assessment of their symptoms, which might not be reliable. Finally, the study focused only on the short-term effects of LSG on weight loss and comorbidity status and detailed information on patient’s dietary habits and physical activity postoperatively were not gathered, and thus, their effect on comorbidities remission rates is unknown. Future studies are required to assess the long-term consequences of LSG on weight and metabolic parameters.
Conclusion
This study showed that LSG plays an important role in achieving significant EWL in Omani patients with morbid obesity; however, there was no statistically significant improvement in the resolution or remission of various comorbidities in relation to the level of EWL. Further studies are needed to determine the long-term effect of LSG on weight loss and obesity-related metabolic diseases.
Disclosure
The authors declare no conflicts of interest. No funding was received for this study. The study abstract was published at the 20th International Symposium on Atherosclerosis 2024, Muscat (https://www.atherosclerosis-journal.com/article/S0021-9150(24)01257-7/pdf).
Acknowledgments
The authors extend their sincere gratitude to the surgical department at the Royal Hospital for their cooperation and assistance in data collection, as well as the information technology (IT) department for granting access to the hospital’s IT system for data collection purposes.
references
- 1. Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol (Lausanne) 2021 Sep;12:706978.
- 2. World Health Organization. Obesity and overweight. 2024 [cited 2023 June 14]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 3. Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022 Aug;133:155217.
- 4. Times of Oman. 60 per cent of adults in Oman are overweight or obese: ministry. 2018 [cited 2023 June 4]. Available from: https://timesofoman.com/article/67846-60-per-cent-of-adults-in-oman-are-overweight-or-obese-ministry.
- 5. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al; GBD 2015 Obesity Collaborators. GBD 2015 obesity collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017 Jul;377(1):13-27.
- 6. Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg 2008 Dec;207(6):928-934.
- 7. Bonsignore MR. Obesity and obstructive sleep apnea. Handb Exp Pharmacol 2022;274:181-201.
- 8. Waters H, DeVol R. Weighing down America: the health and economic impact of obesity. 2016 [cited 2023 June 12]. Available from: https://milkeninstitute.org/report/weighing-down-america-health-and-economic-impact-obesity.
- 9. Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 2013 Oct;347:f5934.
- 10. Roa PE, Kaidar-Person O, Pinto D, Cho M, Szomstein S, Rosenthal RJ. Laparoscopic sleeve gastrectomy as treatment for morbid obesity: technique and short-term outcome. Obes Surg 2006 Oct;16(10):1323-1326.
- 11. Noah J, Smith A, Birch D, Karmali S. The metabolic effects of laparoscopic sleeve gastrectomy: a review. J Minim Invasive Surg Sci 2013;2(3):3-7.
- 12. Schroeder R, Harrison TD, McGraw SL. Treatment of adult obesity with bariatric surgery. Am Fam Physician 2016 Jan;93(1):31-37.
- 13. Lee WJ, Almalki O. Recent advancements in bariatric/metabolic surgery. Ann Gastroenterol Surg 2017 Sep;1(3):171-179.
- 14. AlKhaldi LK, AlSaffar NA, AlHamdan F, Almutairi R, Alipour MH, Haddad EA, et al. Long-term outcomes after laparoscopic sleeve gastrectomy in Kuwait. Ann Saudi Med 2019;39(2):100-103.
- 15. Ruiz-Tovar J, Martínez R, Bonete JM, Rico JM, Zubiaga L, Diez M, et al; Grupo OBELCHE. Grupo OBELCHE. Long-term weight and metabolic effects of laparoscopic sleeve gastrectomy calibrated with a 50-Fr bougie. Obes Surg 2016 Jan;26(1):32-37.
- 16. Salama TM, Sabry K, Eweida GH. Early metabolic outcome after laparoscopic sleeve gastrectomy in morbid obese patients. Surgery Curr Res 2016;6(266):2161-1076.
- 17. Kowalewski PK, Olszewski R, Walędziak MS, Janik MR, Kwiatkowski A, Gałązka-Świderek N, et al. Long-term outcomes of laparoscopic sleeve gastrectomy—a single-center, retrospective study. Obes Surg 2018 Jan;28(1):130-134.
- 18. Golomb I, Ben David M, Glass A, Kolitz T, Keidar A. Long-term metabolic effects of laparoscopic sleeve gastrectomy. JAMA Surg 2015 Nov;150(11):1051-1057.
- 19. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009 Nov;41(4):1149-1160.
- 20. American Diabetes Association. Standards of care in diabetes—2023 abridged for primary care providers. Clin Diabetes 2022;41(1):4-31.
- 21. Jones NR, McCormack T, Constanti M, McManus RJ. Diagnosis and management of hypertension in adults: NICE guideline update 2019. Br J Gen Pract 2020 Jan;70(691):90-91.
- 22. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/american heart association task force on clinical practice guidelines. Circulation 2019 Sep;140(11):e596-e646.
- 23. Khalaj A, Tasdighi E, Hosseinpanah F, Mahdavi M, Valizadeh M, Farahmand E, et al. Two-year outcomes of sleeve gastrectomy versus gastric bypass: first report based on Tehran obesity treatment study (TOTS). BMC Surg 2020 Jul;20(1):160.
- 24. Kikkas EM, Sillakivi T, Suumann J, Kirsimägi Ü, Tikk T, Värk PR. Five-year outcome of laparoscopic sleeve gastrectomy, resolution of comorbidities, and risk for cumulative nutritional deficiencies. Scand J Surg 2019 Mar;108(1):10-16.
- 25. Turgut E, Aydın C, Uğurlu L. The effects of laparoscopic sleeve gastrectomy on metabolic syndrome. Bariatr Surg Pract Patient Care 2019;14(4):172-177.
- 26. Guerreiro V, Neves JS, Salazar D, Ferreira MJ, Oliveira SC, Souteiro P, et al; AMTCO Group. AMTCO Group. Long-term weight loss and metabolic syndrome remission after bariatric surgery: the effect of sex, age, metabolic parameters and surgical technique – a 4-year follow-up study. Obes Facts 2019;12(6):639-652.
- 27. Murshid KR, Alsisi GH, Almansouri FA, Zahid MM, Boghdadi AA, Mahmoud EH. Laparoscopic sleeve gastrectomy for weight loss and treatment of type 2 diabetes mellitus. J Taibah Univ Med Sci 2021 Jan;16(3):387-394.
- 28. Lechea E, Popescu M, Dimulescu D, Godoroja D, Copaescu C. The impact of bariatric surgery on diabetes and other cardiovascular risk factors. Chirurgia (Bucur) 2019;114(6):725-731.
- 29. Al Khayat A, Al Hendi S, Qadhi I, Al Murad A. The effect of laparoscopic sleeve gastrectomy on glycemic control in type 2 diabetic patients. Cureus 2021 Aug;13(8):e16986.
- 30. McTigue KM, Wellman R, Nauman E, Anau J, Coley RY, Odor A, et al; PCORnet Bariatric Study Collaborative. Comparing the 5-year diabetes outcomes of sleeve gastrectomy and gastric bypass: the national patient-centered clinical research network (PCORNet) bariatric study. JAMA Surg 2020 May;155(5):e200087.
- 31. Capoccia D, Coccia F, Guida A, Rizzello M, De Angelis F, Silecchia G, et al. Is type 2 diabetes really resolved after laparoscopic sleeve gastrectomy? Glucose variability studied by continuous glucose monitoring. J Diabetes Res 2015;2015:674268.
- 32. Li Q, Chen L, Yang Z, Ye Z, Huang Y, He M, et al. Metabolic effects of bariatric surgery in type 2 diabetic patients with body mass index < 35 kg/m2. Diabetes Obes Metab 2012 Mar;14(3):262-270.
- 33. Yin X, Qian J, Wang Y, Yang C, Jia B, Cheng Y, et al. Short-term outcome and early effect on blood pressure of laparoscopic sleeve gastrectomy in morbidly obese patients. Clin Exp Hypertens 2019;41(7):622-626.
- 34. Hussein AM. The state of dyslipidemia after laparoscopic sleeve gastrectomy. Int Surg J 2018;5(7):2392-2397.
- 35. Vigilante A, Signorini F, Marani M, Paganini V, Viscido G, Navarro L, et al. Impact on dyslipidemia after laparoscopic sleeve gastrectomy. Obes Surg 2018 Oct;28(10):3111-3115.
- 36. American Academy of Sleep Medicine. Obstructive sleep apnea. Illinois: American Academy of Sleep Medicine; 2008.