| |
Abstract
We describe the case of a 30-year-old male, a known patient of type 1 diabetes mellitus (DM) on insulin therapy, seeking medical attention for recent onset repeated attacks of hypoglycemia associated with generalized weakness and darkening of skin. Further evaluation and screening revealed autoimmune adrenal failure together with presence of Hashimoto’s thyroiditis. The patient was diagnosed as a case of autoimmune polyglandular syndrome (APS) type II with complete triad of Addison’s disease, type 1 DM and autoimmune thyroid disease. Anti-thyroid peroxidase, anti-glutamic acid decarboxylase and anti-endomysial antibodies were present in our patient. He was started on replacement therapy with physiological dose of prednisolone and thyroxine resulting in marked improvement in his symptoms. Recurrent hypoglycemia in a type 1 DM patient should raise a suspicion of underlying autoimmune adrenal insufficiency. Absence of obvious signs of thyroid dysfunction also poses a diagnostic challenge for the clinicians. This article aims at highlighting the importance of detailed evaluation together with long term followup of these patients and their relatives as overt clinical disease may only be the tip of the iceberg of other underlying organ-specific autoimmune diseases that may develop later in the course.
Keywords: Autoimmune polyglandular syndrome; Hypoglycemia; Type 1 diabetes mellitus; Addison’s disease; Autoimmune thyroid disease.
Introduction
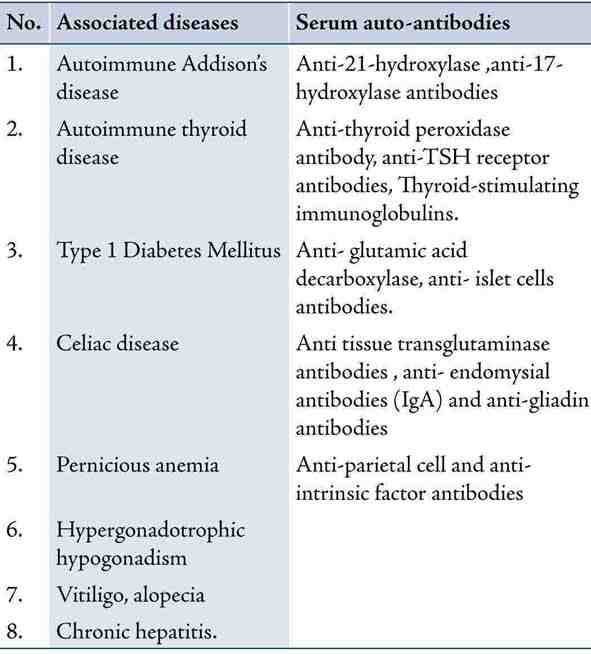
Autoimmune polyglandular syndromes (APS) are a rare group of polyendocrinopathies encompassing multiple endocrine gland insufficiencies associated with other autoimmune diseases resulting from immune-mediated destruction.1 When encountered with a single autoimmune endocrine dysfunction, it is imperative on the part of clinician to consider whether an individual is at risk of having polyglandular syndrome. There are four types of APS described by Neufeld and Blizzard based on clinical grounds.2 Type II APS or Schmidt’s syndrome, is characterized by the obligatory occurrence of autoimmune Addison’s disease (AD) in combination with thyroid autoimmune diseases with or without type 1 DM. These patients require long term followup as they often develop other organ specific autoimmune disorders like hypergonodatropic hypogonadism, vitiligo, chronic atrophic gastritis, pernicious anemia, autoimmune chronic hepatitis, and celiac disease later during the course of the disease. Replacement therapy is the corner stone of treatment of polyglandular syndromes but some caution should be observed. We describe a case of type II APS with complete triad of disease and also associated presence of anti-endomysial antibody without any overt celiac disease which is very unusual.
Case Report
A 30-year-old male farmer, father of one child, presented to the emergency department in an unconscious state. Random capillary blood glucose showed hypoglycemia (51 mg/dL). He recovered with conservative treatment. He was a known type 1 DM patient on twice daily premixed insulin therapy for the last 4 years. For the last few months, he was having recurrent attacks of hypoglycemia requiring his physician to lower the dose of insulin. On further inquiry, he also gave history of generalized weakness, easy fatigability, postural dizziness, gradual darkening of skin and tongue for the last 6 months. The patient denied any history of diarrhea, weight loss, or loss of libido. No history of similar illness in the family was given.
On examination, his heart rate was 96/minute and blood pressure was 90/60 mm of Hg along with orthostatic hypotension. On further examination, there was generalized darkening of skin along with hyperpigmented patches noted on his elbows, palms, knuckles of hands, soles, tongue, oral mucosa including the palates (Fig. 1A, B, C). Thyroid gland was palpable. Secondary sexual characteristics were well developed.
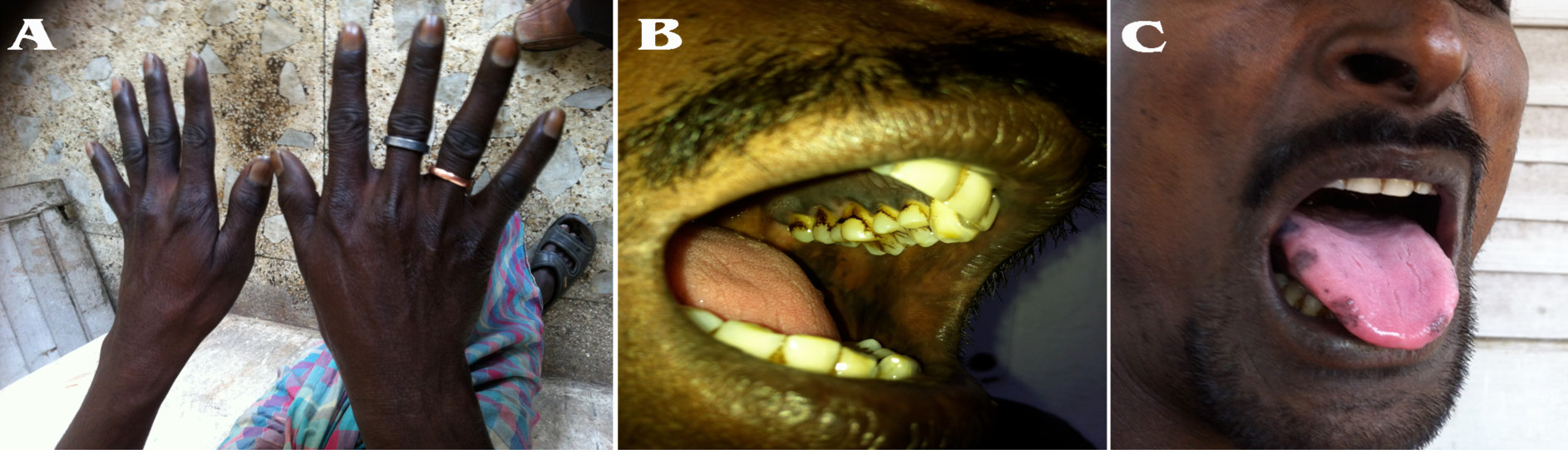
Figure 1: The knuckles of hand, oral mucosa and tongue shows dark hyperpigmented patches suggestive of adrenal insufficiency.
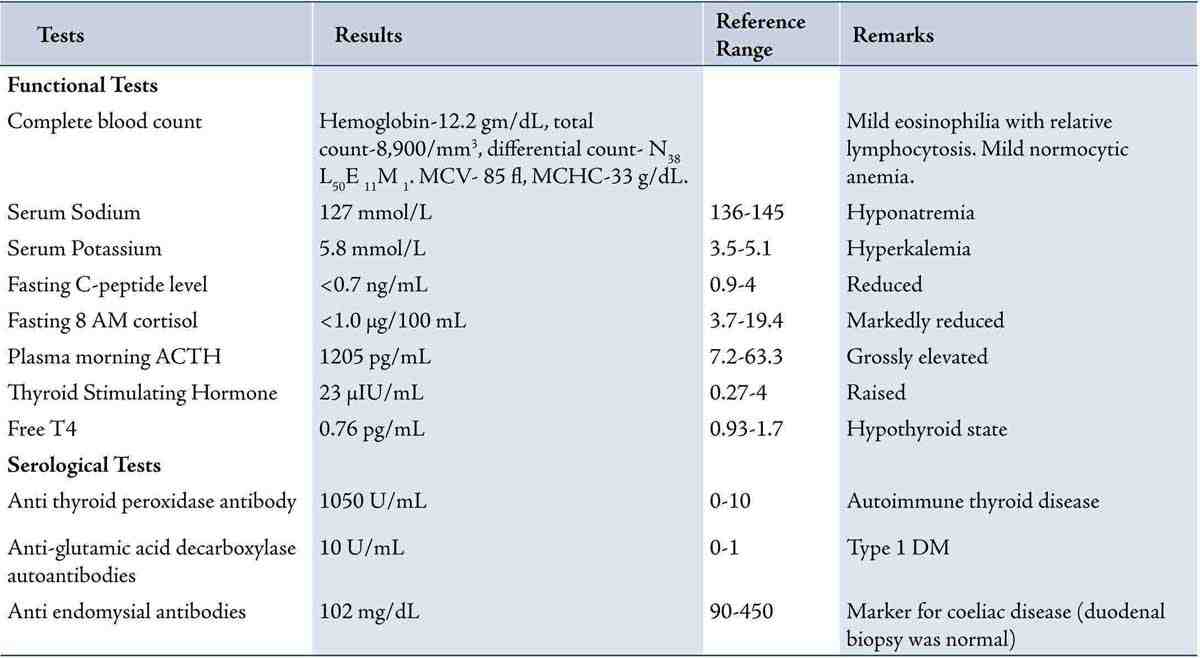
His relevant laboratory investigation reports are summarized in Table 1. Complete blood count showed mild eosinophilia. HbA1c was 5.9% and fasting C-peptide was low. Serum electrolytes showed hyperkalemia with hyponatremia. Having the clinical suspicion of adrenal insufficiency, serum fasting 8 am cortisol measurement was done which showed value of <1.0 µg/dL. His morning plasma ACTH levels were grossly elevated. Having been diagnosed as type 1 DM with Addison’s disease, further endocrine workup was done. It revealed a raised serum TSH value and low plasma free T4 value (Table 1). Serum LH, FSH, prolactin, and testosterone measurements were normal. His chest radiograph was essentially normal. Contrast-enhanced computed tomography (CT) scan of adrenals showed morphologically normal-looking adrenal glands ruling out infective and infiltrative pathology (Fig. 2). Thyroid ultrasound revealed an enlarged gland with a note of diffuse parenchymal disease. High titres of anti-thyroid peroxidase antibodies and anti-glutamic acid decarboxylase antibodies were also established (Table 1). Anti-carboxylase antibodies and anti-21-OH antibodies were not commercially available to us. Anti-endomysial antibodies were positive though upper GI endoscopy with duodenal biopsy was normal. Screening for other endocrine organs was essentially normal. Biochemical screening of the family members for relevant endocrine dysfunction was noncontributory.
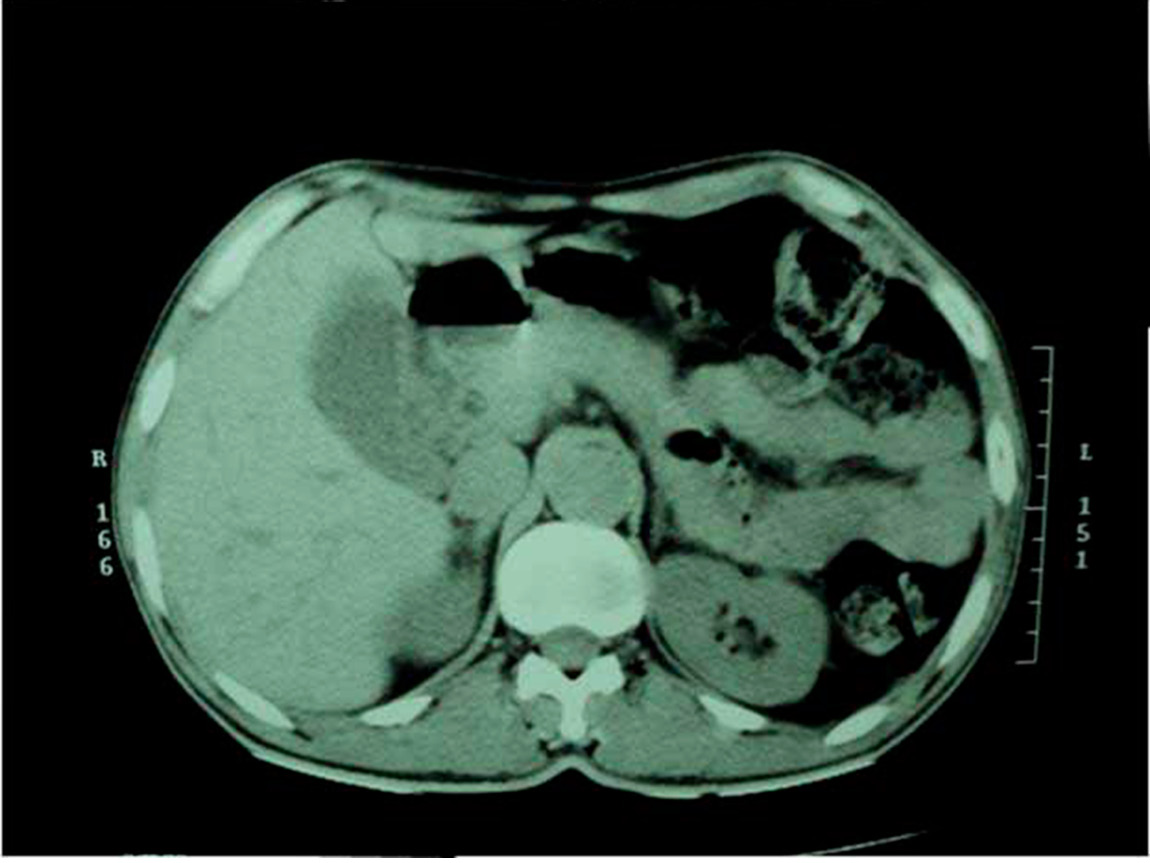
Figure 2: Contrast enhanced CT scan of abdomen shows bilateral morphologically normal adrenal glands.
Table 1: Summarizes the results of diagnostic tests done for the consideration of APS.
He was diagnosed as a case of autoimmune polyglandular syndrome type II or the Schmidt’s syndrome as he was having a triad of type 1 DM, autoimmune Addison’s disease, and Hashimoto’s thyroiditis. His insulin therapy was optimized. Physiological doses of prednisolone (7.5 mg/day) in two divided doses with 2/3 in the morning and 1/3 in the evening were initiated. Levothyroxine at a dose of 1.6 µg/kg body weight/day was started. He was also put on fludrocortisone 0.1 mg/day as his electrolyte abnormality persisted at outdoor visit. The patient showed marked improvement in his symptoms at 3-month followup with normalization of serum electrolytes and thyroid profile. He was advised on the possibility of occurrence of other autoimmune diseases and their manifestations.
Discussion
Autoimmune endocrine gland disorders are usually considered as the tip of the iceberg as they often coexists with other autoimmune gland dysfunction which may not be apparent on first instance. The associations between various autoimmune diseases were found not to appear at random but in particular combinations. In 1980, Neufeld and Blizzard organized and classified these clinical conditions and defined them as polyglandular autoimmune diseases, also termed autoimmune polyendocrine syndromes (APS).2 In APS there is immune mediated destruction of tissues resulting in failure of endocrine as well as non-endocrine organs leading to hypofunctional state.
APS type II, also known as Schmidt’s syndrome, is a rare condition occurring with a prevalence of 1.4-2.0 per 100,000 inhabitants.3 Schmidt found on excisional biopsy presence of lymphocytic infiltration of the adrenal cortex and thyroid gland in a patient who died from adrenal insufficiency in 1926.4 From that time, the coexistence of AD and autoimmune thyroid disease has been referred to as Schmidt’s syndrome. The link between Schmidt’s syndrome and diabetes mellitus was confirmed by Carpenter in his review of 142 patients of Schmidt’s syndrome and the complete triad of Addison’s disease, type 1 DM, autoimmune thyroiditis is also called Carpenter’s syndrome.5 APS type II typically occurs in a middle-aged female with female:male ratio of 2-3.7.6 It is very rare in children. Our patient had all the three major components of APS type II which is present in about 10% of cases only. It is genetically complex and has no clear pattern of inheritance. The various components of APS type II generally occurs in a particular sequence. Type 1 DM in general tends to develop before autoimmune AD; autoimmune thyroid disease develops either before along with or after AD. Bettlere et al.3 have described the prevalence of various components of APS II. The most common was the combination of Addison’s disease and chronic thyroiditis, which occurred in 56.1% of cases. The other combinations were as follows: Addison’s and Graves’ disease (21.2%); Addison’s disease and type 1 diabetes (10.9%); Addison’s disease, chronic thyroiditis, and type 1 diabetes (9.6%); Addison’s disease, Graves’ disease and type 1 diabetes (2.0%).
The pathogenesis of gland destruction in APS is considered to be multifactorial with both environmental and host factor playing interplay to trigger autoimmunity against endocrine glands. Even in the absence of overt disease, presence of organ-specific antibodies points towards ongoing destruction of endocrine tissues. This organ destruction eventually leads to secretory insufficiency and overt clinical disease. Therefore, the clinical presentation of various APS components are preceded by a latent period of months to years which are marked by the presence of circulating organ and cell-specific autoantibodies.1 Suspicion towards presence of autoimmune polyglandular disorder is obtained through history taking and laboratory detection of organ-specific autoantibodies and hormonal deficiencies.
Our patient had type 1 DM and overt features of Addison’s disease. On screening for other autoimmune diseases, autoimmune hypothyroidism was diagnosed in the absence of symptoms of hypothyroidism. Whether to routinely screen for organ specific antibodies in patients with single autoimmune disease is debatable. Dittmar and Kahaly1 in their study have proposed a diagnostic approach towards screening of patients with APS. First the functional screening which includes baseline hormone levels including TSH, FSH, LH, free T4, testosterone, estradiol, fasting morning cortisol, glucose, and complete blood counts and electrolytes should be done. Further, detecting organ-specific autoantibodies by serologic screening indicates the etiology of the disease and identifies patients who may develop autoimmune polyendocrinopathies. These include autoantibodies to islet cells, glutamic acid decarboxylase, IA2, thyroid peroxidase, thyroglobulin, TSH receptor, cytochrome P450 enzymes (21-OH, 17-OH, and side chain cleavage), H+/K+ ATPase of the parietal cells, intrinsic factor, endomysium, transglutaminase and gliadin. Genetic screening, according to the literature, is more useful for APS I than type II. Optional genetic screening tests include molecular analysis of AIRE gene, especially for APS I, and HLA typing and sub typing. Dittmar and Kahaly recommended that for patients with monoglandular autoimmune endocrinopathy, functional screening for APS should be done every 3 years until the age of 75.1 If pathological findings, such as the occurrence of a second autoimmune endocrine disease, are noted, measurement of organ-specific autoantibodies should be added. Furthermore, functional screening for autoimmune endocrine diseases among first-degree relatives of these patients with newly-diagnosed APS may also be done.
Table 2: Summarizes the various possible components of APS 2 and their associated circulating autoantibodies.6
Conclusion
It is important to recognize these clinical settings in type 1 diabetic patients in order to promptly start adequate replacement therapy to prevent potentially serious and life-threatening consequences. One needs to be cautious in managing patients with APS II as initiating thyroxine supplements or modifying insulin dosage without investigating for possible coexistence of underlying adrenal insufficiency may prove to be fatal. Patient education, detailed evaluation, and long term followup for possible co-existence of diseases are the cornerstones for managing patients with APS.
References
1. Dittmar M, Kahaly GJ. Polyglandular autoimmune syndromes: immunogenetics and long-term follow-up. J Clin Endocrinol Metab 2003 Jul;88(7):2983-2992.
2. Neufeld M, Maclaren NK, Blizzard RM. Two types of autoimmune Addison’s disease associated with different polyglandular autoimmune (PGA) syndromes. Medicine (Baltimore) 1981 Sep;60(5):355-362.
3. Betterle C, Lazzarotto F, Presotto F. Autoimmune polyglandular syndrome Type 2: the tip of an iceberg? Clin Exp Immunol 2004 Aug;137(2):225-233.
4. Schmidt MB. Eine biglandulare Erkrankung (Nebennieren und Schilddruse) bei Morbus Addisonii. Verh Dtsch Ges Pathol 1926;21:212-221.
5. Carpenter CC, Solomon N, Silverberg SG, Bledsoe T, Northcutt RC, Klinenberg JR, et al. Schmidt’s syndrome (thyroid and adrenal insufficiency): a review of the literature and a report of fifteen new cases including ten instances of coexistent diabetes mellitus. Medicine (Baltimore) 1964 Mar;43:153-180.
6. Betterle C, Dal Pra C, Mantero F, Zanchetta R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev 2002 Jun;23(3):327-364.
|
|