Uterine fibroids (leiomyoma) are the most common pelvic neoplasm in women, with an estimated lifetime risk of around 70%.1 The prevalence of uterine fibroids ranges from 4.5% to 68.6%, depending on study populations and diagnostic methods.2 A systematic review of common gynecological disorders in the Middle East found a prevalence of 30.6% for fibroids.3
In pathological terms, leiomyoma is classified under uterine smooth muscle tumors. Uterine smooth muscle tumors consist of a spectrum of diseases including of benign uterine leiomyoma (fibroids), fibroid variants (mitotically active, cellular, and atypical leiomyoma), smooth muscle tumors of uncertain malignant potential (STUMP), and malignant leiomyosarcoma.4,5 These conditions can only be differentiated through histopathological examination of surgical specimens.5 These tumors exhibit progressively increasing degrees of cellular pleomorphism characterized by increasing mitosis, atypical cells, abundant eosinophilic cytoplasm, bizarre nuclei, hyperchromatism, multinucleation, irregular nuclear shapes, intra-nuclear inclusions, and coagulative necrosis.5 Uterine sarcomas are rare tumors with an incidence of 1.7 per 100 000 women.6 Although fibroid variants, STUMP, and sarcoma are uncommon in occurrence, these conditions have worse clinical prognosis in terms of recurrence and mortality in comparison to
benign leiomyoma.5
There are surgical and nonsurgical options for the treatment of presumed uterine leiomyoma. Surgical options are myomectomy and hysterectomy, which can be performed using minimally invasive surgery, vaginal surgery, or laparotomy.7 Nonsurgical options include expectant management, pharmacological treatment, magnetic resonance-guided focused ultrasonography, uterine artery embolization, and laparoscopic uterine artery occlusion.7 Women in Oman complete their families in their late reproductive years due to increased female literacy and economic status.8 Advanced age and nulliparity are risk factors for developing fibroid uterus.9 Hence, more women may opt for nonsurgical options and uterus-conserving surgeries for presumed uterine leiomyoma. However, if fibroid variants, STUMP, or sarcoma are suspected pre-operatively, radical surgical treatment with removal of the uterus becomes necessary.
Nonsurgical treatment of presumed uterine leiomyoma does not yield a specimen for a conclusive histopathological diagnosis. Undiagnosed sarcomas have been reported after uterine artery embolization.10,11 According to the Food and Drug Administration, among the women whose presumed uterine leiomyomas are treated surgically, 1 in 580 to 1 in 225 may have hidden uterine sarcoma.12 Minimally invasive surgery may disrupt the uterine mass, which may then lead to the dissemination of malignant cells.13,14 Morcellators are used to retrieve specimens in minimally invasive surgery. Seidman et al,15 found that out of 1091 cases of fibroids which underwent morcellation, an unexpected diagnosis of leiomyoma variants or sarcomas occurred in 1.2% of the cases. The use of laparoscopic power morcellator during minimally invasive surgery in women with hidden uterine sarcomas is associated with reduced chances of long-term survival without cancer.12 Therefore, there have been many studies attempting to identify preoperative predictive factors for the diagnosis of fibroid variants and sarcoma.16,17
Neither risk factors, like age and parity, nor symptomatology can be used to differentiate between sarcoma and leiomyoma. There are mixed findings about age as a potential predictor for sarcoma, with some studies finding positive associations,18,19 and others none.20,21 Altogether, a recent review concluded that age may not be used as an independent predictor to assess the risk of sarcoma.22 A large cross-sectional study found that greater parity was associated with a decreased risk of uterine fibroids.23 On the other hand, Kuisma et al,24 suggested that mechanical forces due to pregnancy and delivery may contribute to chromosomal damage in uterine muscles, increasing the risk of leiomyomas and sarcomas. Though they found parity to be associated with chromosomal damage, they did not find any associations between parity and leiomyosarcoma.24 Another recent large population-based study also found no such association.25 Fibroids and sarcomas present similarly as focal masses in the myometrium with the clinical features of heavy menstrual bleeding, anemia, abdominal mass, pressure symptoms, sub-fertility, and pregnancy complications.7,26,27
If leiomyoma variants, STUMPs, and sarcomas can be identified radiologically, then conservative management of a presumed leiomyoma becomes safer. Eventually, this might reduce healthcare costs. Though some authors reported that radiological findings could predict later histopathological diagnosis,28,29 a recent review concluded that evidence regarding the usefulness of the radiological conclusions was inconclusive.27 A prospective studyof 42 patients awaiting surgery, using magnetic resonance imaging (MRI) to characterize the myoma to its subtypes and sarcoma,28 reported an increased sensitivity of 100% in using diffusion-weighted and post-contrast graphic sequences compared to morphological examination, with a specificity of 88%, a positive predictive value of 66%, a negative predictive value of 100%, and diagnostic accuracy of 90%. Using a sample of 65 women with 105 lesions, Malek et al,29 found that using both the T2 scaled ratio and tumor myometrium contrast-enhanced ratio in preoperative MRI led to a 100% sensitivity and 89% specificity of detecting malignancies. However, Sun et al,27 concluded that there needs to be a greater consensus regarding the usefulness of MRI features and emphasized the need for more studies in this context. Hence, we decided to compare the epidemiological and clinical features of variant leiomyoma to classical leiomyoma and assess their radio-histological correlation.
Methods
We conducted a retrospective observational study to compare the epidemiological and clinical features of classical leiomyoma, variant leiomyoma, STUMP, and sarcoma and to assess their radio-histological correlation.
All patients who underwent surgical treatment for uterine fibroids at the Royal Hospital, Muscat, Oman from 1 January 2011 to 31 December 2016 were included in the study. The Royal Hospital is a tertiary referral center accepting referrals from all over Oman for complex gynecology cases. Ethical approval was obtained from the Ministry of Health Centre for Studies and Research (approval number: SRC #17/2019).
Data collection was done retrospectively from electronic records maintained on the Royal Hospital’s Al Shifa comprehensive healthcare information management system. Demographic details, symptom profiles (heavy menstrual bleeding, dysmenorrhea, subfertility, and anemia defined as hemoglobin (Hb) < 11 g/dL), radiological findings from pictures archiving system, type of surgery, and histopathological reports for all patients were collected. Investigators from the Department of Radiology cross-verified the entries. The histopathological report was used as the diagnostic standard to categorize patients into those with classical leiomyoma, variant leiomyoma, and sarcoma.
The analysis was done using SPSS (IBM Corp. Released 2023. IBM SPSS Statistics for Windows, Version 29.0.2.0 Armonk, NY: IBM Corp). Categorical information was presented using frequency and percentages. For comparing demographic details and symptom profiles of classical leiomyoma, variants, and sarcoma, the chi-square test was used. Radiological findings and histopathological diagnosis were cross-tabulated. As there were only two cases of sarcoma and no cases of STUMP, patients were classified into two groups to assess radio-histological correlation: classical leiomyoma and non-classical varieties, which included variants and sarcoma. The sensitivity and specificity of radiological diagnosis against the standard histopathological diagnosis were calculated. Positive predictive value, negative predictive value, and area under the curve were calculated using MedCalc® Statistical Software version 22.023. A p-value of < 0.05 was considered statistically significant.
Results
During the study period, 14 269 women attended the gynecology outpatient clinic, of which 2780 (19.5%) were clinically diagnosed with a fibroid uterus. Fibroid uterus was managed with surgery in 545 (19.6%) women, and all were included in this study. Of the remaining women, 55 (2.0%) underwent uterine artery embolization, and 2180 (78.4%) were conservatively managed with periodical follow-up. Of the surgically-treated women, myomectomy was done for 390 (71.6%), and hysterectomy for 155 (28.4%). Route of myomectomy was hysteroscopic in eight (2.1%), vaginal in seven (1.8%), laparoscopic in 91 (23.3%), and laparotomy in 284 (72.8%) women. The route of hysterectomy was laparoscopic in 68 (43.9%) women, vaginal in 41 (26.5%) women, and abdominal in 46 (29.7%) women [Figure 1].
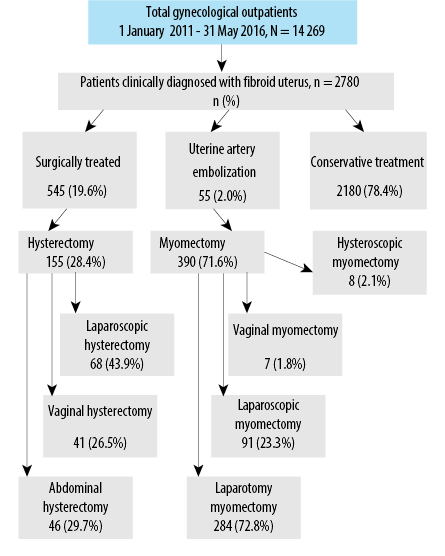 Figure 1: Distribution of patients with clinically diagnosed fibroid uterus according to their management plan.
Figure 1: Distribution of patients with clinically diagnosed fibroid uterus according to their management plan.
The histopathological reports of the surgically treated cases were analyzed; 508 (93.2%) cases were diagnosed as classical leiomyoma, 35 (6.4%) as variant leiomyoma, and two (0.4%) as sarcoma. No cases were diagnosed as STUMP [Table 1].
Table 1: Prevalence of leiomyoma variants according to histopathological reports.
|
Classical
|
508
|
93.2
|
|
Non-classical varieties
|
37
|
6.8
|
|
Variants
|
35
|
6.4
|
The demographic details and clinical features of women with classical leiomyoma, variant leiomyoma, and sarcoma are compared in Table 2. A statistically significant association between age and diagnostic categories was observed by chi-square test (p = 0.032). The youngest age group (15–24 years) accounted for 13 (2.4%) cases, and the histopathological report was classical leiomyoma for all. In the 25–40 years age group, there were 338 (62.0%) cases. Histopathology was reported as classical leiomyoma in 310 (61.0%) cases and variants in 28 (80.0%) cases. In women > 40 years, there were 195 (35.6%) myomas, of which classical leiomyomas were 185 (36.4%), and variants were seven (20.0%). Both the sarcomas were in women aged > 40 years.
Table 2: Comparison of demographic and clinical features between classical leiomyoma, variant leiomyoma, and sarcoma.
|
Age, years
|
|
|
|
|
|
15–24
|
13 (2.4)
|
13 (2.6)
|
0 (0.0)
|
0 (0.0)
|
|
25–40
|
338 (62.0)
|
310 (61.0)
|
28 (80.0)
|
0 (0.0)
|
|
> 40
|
194 (35.6)
|
185 (36.4)
|
7 (20.0)
|
2 (100)
|
|
Marital status
|
|
|
|
|
|
Married
|
409 (75.0)
|
380 (74.8)
|
27 (77.1)
|
2 (100)
|
|
Single
|
135 (24.8)
|
127 (25.2)
|
8 (22.9)
|
0 (0.0)
|
|
Paritya
|
|
|
|
|
|
Nulliparous
|
275 (50.7)
|
259 (51.0)
|
16 (45.7)
|
0 (0.0)
|
|
1–4
|
154 (28.3)
|
137 (27.0)
|
17 (48.6)
|
0 (0.0)
|
|
≥ 5
|
114 (20.9)
|
110 (21.7)
|
2 (5.7)
|
2 (100)
|
|
Symptomsb
|
|
|
|
|
|
HMB
|
280 (51.4)
|
263 (51.8)
|
15 (42.9)
|
2 (100)
|
|
Dysmenorrhea
|
205 (37.6)
|
191 (37.6)
|
14 (40.0)
|
0 (0.0)
|
|
Subfertility
|
78 (14.3)
|
76 (15.0)
|
2 (5.7)
|
0 (0.0)
|
|
Anemia (Hb < 11)
|
238 (43.7)
|
218 (42.9)
|
18 (51.4)
|
2 (100)
|
|
Clinical sizea
|
|
|
|
|
|
Not palpable
|
195 (35.4)
|
186 (36.6)
|
7 (20.0)
|
2 (100)
|
|
Below umbilicus
|
208 (38.2)
|
192 (37.8)
|
16 (45.7)
|
0 (0.0)
|
|
Above umbilicus
|
77 (14.1)
|
71 (14.0)
|
6 (17.1)
|
0 (0.0)
|
|
Imaging modality used
|
|
|
|
|
|
Ultrasound scan
|
395 (72.5)
|
369 (72.6)
|
25 (71.4)
|
1 (50.0)
|
|
MRI
|
150 (27.5)
|
139 (27.4)
|
10 (28.6)
|
1 (50.0)
|
|
Number of myomasa
|
|
|
|
|
|
< 5
|
57 (10.5)
|
50 (76.9)
|
6 (85.7)
|
1 (100)
|
|
5–10
|
12 (2.2)
|
11 (16.9)
|
1 (14.3)
|
0 (0.0)
|
|
> 10
|
4 (0.7)
|
4 (6.2)
|
0 (0.0)
|
0 (0.0)
|
|
Size of the largest myomaa
|
|
|
|
|
|
< 5 cm
|
30 (5.5)
|
27 (20.0)
|
2 (25.0)
|
1 (100)
|
|
5–10 cm
|
70 (12.8)
|
65 (48.1)
|
5 (62.5)
|
0 (0.0)
|
|
> 10 cm
|
44 (8.1)
|
43 (31.9)
|
1 (12.5)
|
0 (0.0)
|
|
Location of myomaa
|
|
|
|
|
|
Intramural
|
24 (4.4)
|
19 (14.5)
|
4 (50.0)
|
1 (100)
|
|
Submucous
|
8 (1.5)
|
7 (5.3)
|
1 (12.5)
|
0 (0.0)
|
|
Subserous
|
33 (6.1)
|
32 (24.4)
|
1 (12.5)
|
0 (0.0)
|
aMissing values present due to deficient documentation in case files; bPercentages may not add up to 100 as multiple symptoms may be present.
HMB: heavy menstrual bleeding; Hb: hemoglobin; MRI: magnetic resonance imaging.
The chi-square test showed a statistically significant difference between parity and diagnostic groups (p = 0.002). Nulliparous women accounted for 259 (51.0%) cases of classical leiomyoma and 16 (45.7%) variants. Women with parity 1–4 contributed to 137 (27.0%) cases of classical leiomyoma and 17 (48.6%) variants. There was no sarcoma in both of these groups, as both the women diagnosed with sarcoma had parity over five. In women with parity of more than five, classical leiomyoma and variants were 110 (21.7%) and two (5.7%), respectively.
There were no significant differences between the three groups of women in the prevalence of heavy menstrual bleeding, dysmenorrhea, subfertility, and anemia (p-value > 0.148). Overall, 280 (51.4%) women presented with heavy menstrual bleeding, 238 (43.7%) women had anemia, 205 (37.6%) reported dysmenorrhea, and 78 (14.3%) reported subfertility. There was no statistically significant difference between the three groups in the clinical size of the uterus (p = 0.127). Both women diagnosed with sarcoma presented with non-palpable uteri.
Ultrasound scan was the only imaging modality used in 395 (72.8%) cases whereas MRI was also done in 150 (27.5%). The number, size, and location of myomas were not statistically different between groups. The number of myomas was documented as < 5 five in 57 (10.5%), 5–10 in 12 (2.2%), and > 10 in four (0.7%) women. Myomas of > 10 cm were documented in 44 (8.1%) women. Myomas were documented as intramural in 24 (4.4%) women, submucous in eight (1.5%) women, subserous in 33 (6.1%) women, and multiple locations in 75 (13.8%) women [Table 2].
The MRI and histopathology reports are cross-tabulated in Table 3. As the number of sarcomas in this cohort was only two, for radio-histological correlation, they were grouped along with variant leiomyomas under non-classical varieties. Out of the 139 cases of classical leiomyoma, MRI correctly identified 125 (89.9%) cases. Fourteen (10.1%) cases were suspected to be non-classical varieties on MRI, but they were classical leiomyomas. MRI correctly predicted 36.4% cases of non-classical leiomyoma but did not identify 63.6% cases.
Table 3: Cross-tabulation for MRI and histopathology reports.
|
MRI report
|
Classical leiomyoma
|
125 (89.9)
|
7 (63.6)
|
Non-classical varieties include variant leiomyoma and sarcoma.
The radio-histological correlation is detailed in Table 4. We found that the sensitivity (95% CI) of MRI to predict classical leiomyoma was 89.93% (83.68–94.38), and its specificity was 36.36% (10.93–69.21). For non-classical varieties, the sensitivity was 22.22% (2.81–60.01), and the specificity was 88.65% (82.23–93.37). MRI has a positive predictive value of 94.70% (91.93–96.55), and a negative predictive value of 22.22% (10.17–41.91) for classical leiomyoma. For non-classical varieties, the positive predictive value was 11.11% (3.27–31.58), and the negative predictive value was 94.70% (92.61–96.22). The positive likelihood ratio and negative likelihood ratio for classical leiomyoma are 1.41 (0.90–2.22), and 0.28 (0.11–0.70), respectively. For non-classical varieties, the positive likelihood ratio was 1.96 (0.53–7.23) and the negative likelihood ratio was 0.88 (0.62–1.25). The area under the curve for classical and variant leiomyoma was 0.63 (0.55–0.71) and 0.55 (0.47–0.64). The accuracy of MRI in predicting classical leiomyoma was 86.00 (79.40–91.12) and the non-classical varieties was 84.67 (77.87–90.03).
Table 4: The radio-histological correlation of MRI to classical (n = 139) and variant (n = 11) leiomyoma.
|
Classical leiomyoma
|
89.93
(83.68–94.38)
|
36.36
(10.93–69.21)
|
0.63 (0.55–0.71)
|
1.41
(0.90–2.22)
|
0.28
(0.11–0.70)
|
94.70 (91.93–96.55)
|
22.22 (10.17–41.91)
|
86.00 (79.40–91.12)
|
Discussion
This study retrospectively examined the radio-histological correlation of classical and variant leiomyomas in all patients who underwent surgical treatment of fibroids. MRI could successfully predict 89.9% of classical leiomyoma and could predict only 36.4% of non-classical varieties (variant leiomyoma, STUMP, and sarcoma).
Among the women who attended the gynecology outpatient department of the Royal Hospital, 2780 (19.5%) were diagnosed as having a fibroid uterus. This prevalence rate is comparable to those found in other hospital-based samples from this region. A similar retrospective study from Saudi Arabia found a prevalence of 21.2% for uterine fibroids,30 and a systematic review of common gynecological disorders in the Middle East found a prevalence of 30.6% (95% CI: 24.9–36.7) for fibroids.3
Of the 545 surgically treated women, we found variant leiomyoma in 35 (6.4%) and sarcoma in two (0.4%), which is comparable to other studies. A 13-year study from a hospital in Turkey found a similar prevalence of variant leiomyoma, where 5.65% (185/3275) of cases were variants.31 Uterine sarcomas have incidence rates ranging from 0.1% to 0.49% in women undergoing surgery for presumed uterine leiomyoma.32,33 A large population-based study in Minnesota, USA, over 14 years, also found the incidence of unexpected sarcomas to be 0.39% (1 in 256 surgeries).33
We observed that age and parity showed differences between classical leiomyoma, variants, and sarcoma. Fibroids and variants were found more in the 25–40-year age group. Edzie et al,34 in a retrospective cohort study from Ghana, stated similarly that women in their prime reproductive ages are most affected by fibroids. In our cohort, both women with sarcoma were over 40 years old. Multinu et al,33 reported that > 45 years of age is a risk factor for sarcoma. However, Żak et al,22 concluded that age cannot be used as an independent predictor for sarcoma. Fibroid variants and sarcomas were found to be more prevalent in parous women in this study. A population-based analysis of 399 uterine leiomyosarcoma patients and 1657 matched controls did not show a statistically significant association between parity and leiomyosarcoma (odds ratio = 1.03; 95% CI: 0.96–1.11).24 A retrospective case-control study conducted in Thailand included 18 218 women who underwent surgical treatment of fibroid uterus, also found no such association.25 Results of the current study were different, probably due to differences in study design and our small sample size.
Heavy menstrual bleeding, anemia, subfertility, and dysmenorrhea were the main presenting features, and there were no significant differences in symptoms between classical leiomyoma, variant leiomyoma, and sarcoma. Other studies also found no differences in symptoms.22,27 We did not find any relationship between the clinical size or the location of fibroids and the histopathological diagnosis. Contrary to our observation, Multinu et al,33 found that the incidence of sarcoma progressively increased with increasing uterine weight from 0.09% in women with uterine weight < 250 g to 0.7%, 1.4%, 6.3%, 11.1%, and 14.3%, in women with uterine weight 250–499, 500–999, 1000–1499, 1500–1999, and ≥ 2000 g, respectively (p < 0.001). Bacanakgil et al,31 reported a higher incidence of myxoid leiomyoma, STUMP, and vascular leiomyoma in patients with a tumor diameter of ≥ 10 cm. Zhang et al,35 also observed that a tumor size ≥ 7 cm was an independent predictor of uterine leiomyosarcoma. Chen et al,36 compared 66 cases of hysterectomy for uterine sarcoma with 66 cases of hysterectomy for benign fibroid, and found that sarcoma was associated with sub-serosal rapidly-growing solitary fibroid.
In the current study, the sensitivity of MRI in predicting non-classical leiomyomas was low, but specificity was high. Good specificity and a good negative predictive value for MRI in non-classical varieties suggest that ruling out a non-classical variant through MRI is relevant to the clinical decision. However, both the positive (1.96; 95% CI: 0.53–7.23) and negative likelihood ratios (0.88; 95% CI: 0.62–1.25) suggest that MRI test results confer no significant changes to the post-test probability of non-classical varieties. The area under the curve of 0.55 suggests that the test has weak discriminatory power to distinguish the non-classical variety from classical leiomyoma. A recent review on the utility of MRI features to distinguish between classical and non-classical leiomyoma concludes that current evidence is mixed.27 However, some studies report achieving a sensitivity of 100% and good specificity for preoperative MRI to predict later histopathological diagnosis.28,29
The challenges in preoperative prediction of leiomyoasarcoma was explained in a review article by Yang et al.37 Similar clinical presentations and distinctly different management strategies for classical leiomyoma and other histological types, including sarcoma, pose a significant clinical dilemma for physicians and patients. Patients are at risk of receiving unnecessary invasive, non-fertility sparing surgical treatments for benign leiomyoma and conservative or less invasive surgery for uterine leiomyoasarcoma, resulting in upstaging malignancy and lower disease-free survival.
Our study is unique as we compared the three aspects of the disease, clinical presentation, radiological findings, and histopathological diagnosis, and we assessed the accuracy of MRI in distinguishing between classical and non-classical varieties of leiomyoma. We are limited by the fact that this was a retrospective single-center study with a limited number of participants. As data collection was done by retrospective record review, deficiencies in documentation might have affected results. We cannot be certain that subtle observer variations in histopathological examination affected the results. Distribution of the sample sizes over the leiomyoma types may be a limitation while testing associations.
Conclusion
The epidemiological data and clinical features did not help distinguish between classical leiomyoma and non-classical varieties. In this study, MRI was found to have low sensitivity, but high specificity in predicting variant leiomyoma and sarcoma. MRI has a weak discriminatory power to distinguish between classical and non-classical leiomyoma. Prospective studies including larger populations, multivariate analysis, and incorporation of advanced MRI parameters with artificial intelligence might be required to ensure more accurate preoperative prediction of variants and sarcoma. Incorporation of the evolving biochemical and genetic parameters17 and validating evolving scoring systems31 also might improve the preoperative prediction of variant leiomyoma, STUMP, and sarcoma.
Disclosure
The authors declare no conflicts of interest. No funding was received for this study.
Acknowledgments
We would like to thank the Pathology, Radiology, and Statistics departments of OMSB for their help in completing this study.
references
- 1. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003 Jan;188(1):100-107.
- 2. Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG 2017 Sep;124(10):1501-1512.
- 3. Mousa M, Al-Jefout M, Alsafar H, Kirtley S, Lindgren CM, Missmer SA, et al. Prevalence of common gynecological conditions in the Middle East: systematic review and meta-analysis. Front Reprod Health 2021 Apr;3:661360.
- 4. Solomon LA, Schimp VL, Ali-Fehmi R, Diamond MP, Munkarah AR. Clinical update of smooth muscle tumors of the uterus. J Minim Invasive Gynecol 2005;12(5):401-408.
- 5. Arleo EK, Schwartz PE, Hui P, McCarthy S. Review of leiomyoma variants. AJR Am J Roentgenol 2015 Oct;205(4):912-921.
- 6. Harlow BL, Weiss NS, Lofton S. The epidemiology of sarcomas of the uterus. J Natl Cancer Inst 1986 Mar;76(3):399-402.
- 7. Vilos GA, Allaire C, Laberge PY, Leyland N, Vilos AG, Murji A, et al; Special Contibutors. The management of uterine leiomyomas. J Obstet Gynaecol Can 2015 Feb;37(2):157-178.
- 8. Islam MM. Rapid fertility decline in Oman: understanding the role of proximate determinants. Middle East Fertil Soc J 2017 Dec;22(4):275-284.
- 9. Yang Q, Ciebiera M, Bariani MV, Ali M, Elkafas H, Boyer TG, et al. Comprehensive review of uterine fibroids: developmental origin, pathogenesis, and treatment. Endocr Rev 2022 Jul;43(4):678-719.
- 10. Al-Badr A, Faught W. Uterine artery embolization in an undiagnosed uterine sarcoma. Obstet Gynecol 2001 May;97(5 Pt 2):836-837.
- 11. Papadia A, Salom EM, Fulcheri E, Ragni N. Uterine sarcoma occurring in a premenopausal patient after uterine artery embolization: a case report and review of the literature. Gynecol Oncol 2007 Jan;104(1):260-263.
- 12. U.S. Food and Drug Administration. Laparoscopic power morcellators. 2023 [cited 2023 August 28]. Available from: https://www.fda.gov/medical-devices/surgery-devices/laparoscopic-power-morcellators.
- 13. Park JY, Park SK, Kim DY, Kim JH, Kim YM, Kim YT, et al. The impact of tumor morcellation during surgery on the prognosis of patients with apparently early uterine leiomyosarcoma. Gynecol Oncol 2011 Aug;122(2):255-259.
- 14. Mowers EL, Skinner B, McLean K, Reynolds RK. Effects of morcellation of uterine smooth muscle tumor of uncertain malignant potential and endometrial stromal sarcoma: case series and recommendations for clinical practice. J Minim Invasive Gynecol 2015;22(4):601-606.
- 15. Seidman MA, Oduyebo T, Muto MG, Crum CP, Nucci MR, Quade BJ. Peritoneal dissemination complicating morcellation of uterine mesenchymal neoplasms. PLoS One 2012;7(11):e50058.
- 16. Valletta R, Corato V, Lombardo F, Avesani G, Negri G, Steinkasserer M, et al. Leiomyoma or sarcoma? MRI performance in the differential diagnosis of sonographically suspicious uterine masses. Eur J Radiol 2024 Jan;170:111217.
- 17. Liu J, Wang Z. Advances in the preoperative identification of uterine sarcoma. Cancers (Basel) 2022 Jul;14(14):3517.
- 18. Rodriguez AM, Zeybek B, Asoglu MR, Sak ME, Tan A, Borahay MA, et al. Incidence of occult leiomyosarcoma in presumed morcellation cases: a database study. Eur J Obstet Gynecol Reprod Biol 2016 Feb;197:31-35.
- 19. Brohl AS, Li L, Andikyan V, Običan SG, Cioffi A, Hao K, et al. Age-stratified risk of unexpected uterine sarcoma following surgery for presumed benign leiomyoma. Oncologist 2015 Apr;20(4):433-439.
- 20. Bi Q, Xiao Z, Lv F, Liu Y, Zou C, Shen Y. Utility of clinical parameters and multiparametric MRI as predictive factors for differentiating uterine sarcoma from atypical leiomyoma. Acad Radiol 2018 Aug;25(8):993-1002.
- 21. Kiliç C, Yuksel D, Cakir C, Turkmen O, Kimyon Comert G, Başaran D, et al. Primary leiomyosarcoma of the uterine cervix: report of 4 cases, systematic review, and meta-analysis. Tumori 2020 Oct;106(5):413-423.
- 22. Żak K, Zaremba B, Rajtak A, Kotarski J, Amant F, Bobiński M. Preoperative differentiation of uterine leiomyomas and leiomyosarcomas: current possibilities and future directions. Cancers (Basel) 2022 Apr;14(8):1966.
- 23. Parazzini F. Risk factors for clinically diagnosed uterine fibroids in women around menopause. Maturitas 2006 Sep;55(2):174-179.
- 24. Kuisma H, Bramante S, Rajamäki K, Sipilä LJ, Kaasinen E, Kaukomaa J, et al. Parity associates with chromosomal damage in uterine leiomyomas. Nat Commun 2021 Sep;12(1):5448.
- 25. Chantasartrassamee P, Kongsawatvorakul C, Rermluk N, Charakorn C, Wattanayingcharoenchai R, Lertkhachonsuk AA. Preoperative clinical characteristics between uterine sarcoma and leiomyoma in patients with uterine mass, a case-control study. Eur J Obstet Gynecol Reprod Biol 2022 Mar;270:176-180.
- 26. D’Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol 2010 Jan;116(1):131-139.
- 27. Sun S, Bonaffini PA, Nougaret S, Fournier L, Dohan A, Chong J, et al. How to differentiate uterine leiomyosarcoma from leiomyoma with imaging. Diagn Interv Imaging 2019 Oct;100(10):619-634.
- 28. Tamburrini O, Smiraglio C, Caputo N, Mastroianni N, Guarascio G, Annalisa DC, et al. The contribution of magnetic resonance imaging in the differential diagnosis between leiomyoma typical, atypical and uterine sarcomas: personal experience. International Journal of Diagnostic Imaging. 2016 Jul;3(2).
- 29. Malek M, Rahmani M, Seyyed Ebrahimi SM, Tabibian E, Alidoosti A, Rahimifar P, et al. Investigating the diagnostic value of quantitative parameters based on T2-weighted and contrast-enhanced MRI with psoas muscle and outer myometrium as internal references for differentiating uterine sarcomas from leiomyomas at 3T MRI. Cancer Imaging 2019 Apr;19(1):20.
- 30. Abbas HY, Awad IA, Alharbi E, Alaameri H, Althubaiti S, Ashkar L. Prevalence and incidence of uterine fibroid at King Abdulaziz University Hospital Saudi Arabia. Clin Med Diagn 2016;6(3):45-48.
- 31. Bacanakgil BH, Ilhan G, Kaban I. Variant type of leiomyomas: 13 years of experience in a single institution. Ginekol Pol 2022;93(6):444-449.
- 32. Odejinmi F, Agarwal N, Maclaran K, Oliver R. Should we abandon all conservative treatments for uterine fibroids? The problem with leiomyosarcomas. Womens Health (Lond) 2015 Mar;11(2):151-159.
- 33. Multinu F, Casarin J, Tortorella L, Huang Y, Weaver A, Angioni S, et al. Incidence of sarcoma in patients undergoing hysterectomy for benign indications: a population-based study. Am J Obstet Gynecol 2019 Feb;220(2):179.e1-179.e10.
- 34. Edzie EK, Dzefi-Tettey K, Brakohiapa EK, Quarshie F, Ken-Amoah S, Cudjoe O, et al. Age of first diagnosis and incidence rate of uterine fibroids in Ghana. A retrospective cohort study. PLoS One 2023 Mar;18(3):e0283201.
- 35. Zhang G, Yu X, Zhu L, Fan Q, Shi H, Lang J. Preoperative clinical characteristics scoring system for differentiating uterine leiomyosarcoma from fibroid. BMC Cancer 2020 Jun;20(1):514.
- 36. Chen I, Firth B, Hopkins L, Bougie O, Xie R hua, Singh S. Clinical characteristics differentiating uterine sarcoma and fibroids. JSLS 2018;22(1):e2017.00066.
- 37. Yang Q, Madueke-Laveaux OS, Cun H, Wlodarczyk M, Garcia N, Carvalho KC, et al. Comprehensive review of uterine leiomyosarcoma: pathogenesis, diagnosis, prognosis, and targeted therapy. Cells 2024 Jun;13(13):1106.