Pericardial-pleural fistula (PPF) is a communication between the pericardium and pleural space, resulting in the movement of pericardial fluid from the pericardial sac into the lower-pressure pleural space.1 PPF may complicate between 0.8–6% of echocardiography-guided pericardiocentesis procedures.2,3 In this case report, we report a 22-year-old male who developed pericardial effusion due to hyperparathyroidism, complicated by pericardial-pleural fistula.
Case report
A 22-year-old male presented to the general medical ward with an underlying end-stage renal disease complicated by secondary hyperparathyroidism with worsening left pleuritic chest pain and shortness of breath for one week. He denied having any cough, fever, night sweats, or constitutional symptoms. A chest radiograph on admission revealed a globular cardiac shadow with widened cardiothoracic ratio. The electrocardiogram showed no evolving changes, and serum troponin was not raised. On examination, he had a blood pressure of 146/71 mmHg, a pulse rate of 88 beats per minute, oxygen saturation of 91% under room air, and was afebrile. His jugular venous pressure was raised, and a cardiac auscultation revealed a pericardial rub. Point-of-care echocardiogram demonstrated global pericardial effusion with no right ventricular collapse, while tlung ultrasound revealed multiple vertical reverberation artifacts, also known as B-lines, but no pleural effusion. He was admitted for oxygen supplementation and decongestion therapy. Given the raised inflammatory markers (C-reactive protein = 76.2 mg/L), antibiotics were commenced empirically to treat pneumonia. However, blood culture, sputum culture, and sputum smear for acid-fast bacilli were all negative. Informed consent was obtained from the patient.
Formal transthoracic echocardiography on day three of admission revealed mitral annular calcification with an extension into the left ventricular outflow tract, not causing obstruction, with pericardial effusion measuring two centimeters maximally without evidence of tamponade [Figure 1]. On day seven of admission, repeat bedside an echocardiography revealed a similar pericardial effusion. In view of a persistent tachycardia and due to suspicion of tuberculous pericarditis, echocardiography-guided pericardiocentesis through the apical approach was performed, revealing hemoserous pericardial fluid. Emergent pericardial fluid investigations revealed protein of 46 g/L (reference range protein exudate: > 25 g/L) and lactate dehydrogenase of 1237 IU/L (reference range lactic acid dehydrogenase exudate: > 200 IU/L), which were suggestive of an exudative effusion. Post-pericardiocentesis, the patient complained of worsening shortness of breath and required increasing oxygen supplementation. Repeated chest radiograph showed a new left pleural effusion with no pneumothorax [Figure 2a].
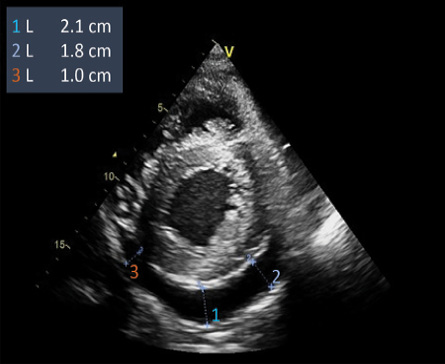 Figure 1: The echocardiogram (parasternal short-axis view) demonstrates a global pericardial effusion deepest posteriorly.
Figure 1: The echocardiogram (parasternal short-axis view) demonstrates a global pericardial effusion deepest posteriorly.
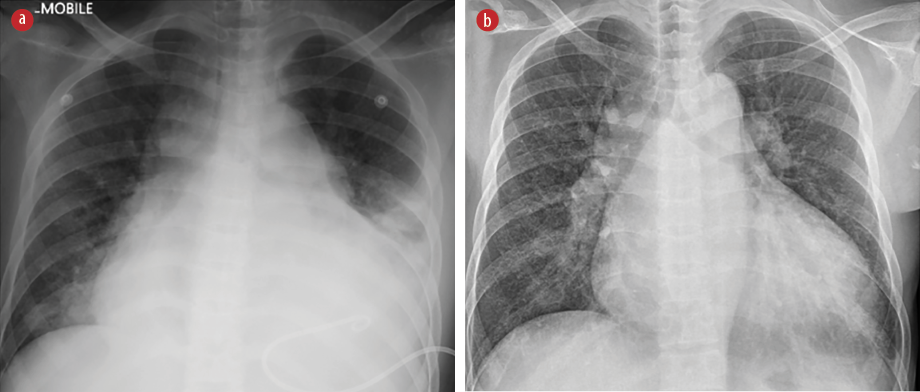 Figure 2: (a) Post-pericardiocentesis chest radiograph, which demonstrated a new left pleural effusion. (b) Follow-up chest radiograph showed no recurrence of pleural effusion.
Figure 2: (a) Post-pericardiocentesis chest radiograph, which demonstrated a new left pleural effusion. (b) Follow-up chest radiograph showed no recurrence of pleural effusion.
A bedside scan showed a new left pleural effusion up to the upper midzone, with the absence of pericardial effusion. A thoracocentesis and pigtail drain insertion were performed. Pleural fluid gross appearance and biochemical picture correlated well with the pericardial fluid, which was hemoserous in color and exudative. The pleural fluid lactic acid dehydrogenase was 1123 IU/L, protein was 45 g/L, and adenosine deaminase was 21.67 U/L (cut-off value pleural adenosine deaminase was 29.6U/L). The cytology and culture were negative for malignant cells, and no organisms were isolated. Following thoracocentesis, the patient was successfully weaned to room air and discharged. Pericardial and pleural fluid tuberculosis cultures were both negative. A repeat chest radiograph six weeks post-thoracocentesis revealed no recurrence of pleural effusion [Figure 2b].
A bacterial, malignant, and tuberculous effusion was ruled out. The echocardiogram findings of mitral annular calcification and serially increasing intact parathyroid hormone levels [Table 1], a final diagnosis of secondary hyperparathyroidism-related pericardial effusion, which was made with multidisciplinary input from the cardiology team.
Table 1: Serial biochemical investigations revealed increasing intact parathyroid hormone (iPTH l) levels
over time.
|
Sodium, mmol/L
|
135–145
|
134
|
133
|
135
|
132
|
|
Potassium, mmol/L
|
3.5–5.1
|
3.5
|
3.8
|
3.6
|
4.1
|
|
Urea, mmol/L
|
2.5–6.7
|
17.3
|
16.5
|
17.6
|
18.3
|
|
Creatinine, umol/L
|
54.0–98.0
|
698.9
|
722.4
|
758.2
|
742.5
|
|
iPTH, pg/ml
|
15.0–68.3
|
435
|
782
|
659
|
1473
|
|
Calcium, mmol/L
|
2.10–2.55
|
2.12
|
2.16
|
2.12
|
2.10
|
Discussion
This is the first reported incidence of PPF in the setting of secondary hyperthyroidism. Previous reports of post-pericardiocentesis PPF have been scarce, with only four case reports and one case series documented.1–4 Among these, two case reports noted the occurrence of pleural effusion on the left side,1,4 while the laterality of the pleural effusions in the remaining two reports was unspecified.2,3 One of the cited risk factors that may have led to PPF in our case was the apical approach. This is due to the adjacent pleural space, which is absent in the subxiphoid approach.3 In one instance, it was suggested that the use of a rigid dilator for pericardial drain placement may have inadvertently traversed an area of pleuro-pericardial overlap, leading to PPF.4
Aside from its association with pericardiocentesis, PPF has also been observed in cardiac procedures such as epicardial catheter ablation for ventricular tachycardia,5 implantable cardioverter-defibrillator insertion,6 or as a result of stab injuries penetrating the right ventricle.7
The prompt identification of PPF is critical in preventing repeated attempts to aspirate an already empty pericardial sac, which could lead to severe myocardial or coronary vessel injury.1 Since there are currently no established technical methods to prevent PPF, maintaining a high level of vigilance and early detection of PPF complications are essential.3
Pericardial effusion associated with secondary hyperparathyroidism was first reported in 1985 in Japan8 and may coexist with a calcified pericardium.9 The calcification is attributed to extraskeletal calcification resulting from secondary hyperparathyroidism.9 Two proposed pathophysiological pathways include alterations in calcium metabolism resulting in ectopic calcification or dystrophic calcification causing tissue damage.10 Additionally, pericardial calcification may also be attributed to hemodialysis, as evidencing in cases where patients exhibit calcified pericardium despite normal serum phosphate, calcium, and parathyroid hormone levels.9
In our patient, we posit that the calcified pericardium was less malleable, rendering it more susceptible to fistula formation. The insertion of the pericardiocentesis needle may have created a residual ‘flap’ that was unable to seal due to its rigid, calcified nature. Furthermore, the apical approach may have caused the needle to traverse through an area encompassing both the pleura and pericardium.
Conclusion
Clinicians should exercise heightened vigilance when performing pericardiocentesis on patients with underlying hyperparathyroidism. Further research is warranted to elucidate predictive factors for PPF and develop strategies to mitigate the risk of this complication.
Disclosure
The authors declare no conflicts of interest.
Acknowledgments
The authors would like to thank the cardiology team, of the Queen Elizabeth Hospital II, for their input in this case.
references
- 1. Winter M, Lim I, Joseph M. The disappearing pericardial effusion: a pericardial-pleural fistula. J Am Soc Echocardiogr 2009 Aug;22(8):973.e5-973.e7.
- 2. Tsang TS, Enriquez-Sarano M, Freeman WK, Barnes ME, Sinak LJ, Gersh BJ, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc 2002 May;77(5):429-436.
- 3. Lau GT, Gillebert C, Perry R, Swan A, Joseph MX. Pericardial-pleural fistula: an underrecognized but surprisingly common and clinically important complication during echocardiography guided pericardiocentesis. J Am Soc Echocardiogr 2019 Mar;32(3):425-427.
- 4. Liebenberg J, van der Bijl P. A “vanishing”, tuberculous, pericardial effusion. Korean Circ J 2016 Nov;46(6):879-881.
- 5. Mathuria N, Buch E, Shivkumar K. Pleuropericardial fistula formation after prior epicardial catheter ablation for ventricular tachycardia. Circ Arrhythm Electrophysiol 2012 Feb;5(1):e18-e19.
- 6. Kiuchi MG, Lobato GM, Chen S. Extraction of a dual-chamber pacemaker and inserting of a new automatic implantable cardioverter defibrillator: the easy procedure almost became catastrophic: a case report. Medicine (Baltimore) 2017 Sep;96(35):e7919.
- 7. Ochi A, Hardikar AA. Delayed presentation of cardiac injury four weeks after stabbing. Asian Cardiovasc Thorac Ann 2020 Jan;28(1):62-64.
- 8. Nogami A, Fujiwara T, Kiso A, Masaki H, Nakai M, Yamane H, et al. A case of chronic constrictive pericarditis with secondary hyperparathyroidism undergoing long-term hemodialysis. The Journal of the Japanese Practical Surgeon Society 1985 Jun 25;46(6):797-803.
- 9. Park EA, Lee W, Kim KH, Chung JW, Park JH. Rapid progression of pericardial calcification containing a “calcium paste” in a patient with end-stage renal disease. Circulation 2011 Mar;123(9):e262-e264.
- 10. Catellier MJ, Chua GT, Youmans G, Waller BF. Calcific deposits in the heart. Clin Cardiol 1990 Apr;13(4):287-294.