Vitamin D plays a crucial role in various physiological processes, including bone health, immune function, and modulation of inflammatory responses.1,2 Neonatal prognosis pertains to the likelihood of a newborn surviving and maintaining good health during the initial 28 days of life. In 2020, the decrease in global mortality rates for children under five years old was more significant than the decline in neonatal mortality rates. Almost half (47%) of all deaths among children < 5 occurred during the newborn period, which is defined as the first 28 days of life.3
The majority of neonatal deaths can be attributed to factors such as preterm birth, complications during delivery (like birth asphyxia), infections, or congenital defects. Most infants who die within the first 28 days of life succumb to conditions and diseases that result from insufficient quality care at birth and during the early days of life.4 Ensuring high-quality antenatal care, skilled care during childbirth, postnatal care for mothers and infants, and specialized care for small and sick newborns can significantly enhance the survival and health of newborns. This approach can also help prevent avoidable stillbirths. Women who receive midwife-led continuity of care have a 16% lower risk of losing a baby and a 24% lower risk of experiencing preterm birth.5
Vitamin D is crucial in maintaining healthy bones and teeth, and its importance is heightened during pregnancy. Vitamin D deficiency is common during pregnancy and can result in abnormal bone growth, fractures, or even newborn rickets. Several studies have associated vitamin D deficiency with an increased risk of complications during pregnancy, such as gestational diabetes, preeclampsia, preterm birth, and low birth weight.6 Low levels of vitamin D during pregnancy are linked to a range of health issues and various consequences, from the preimplantation stage of the fetus to diseases in adulthood. It is currently recognized that a vitamin D deficiency in the mother can impact maternal and fetal calcium homeostasis. This deficiency also affects the development of the fetus’s bones.7 Due to the rapid growth and development of the fetus, particularly the calcification of bones towards the end of pregnancy, pregnant mothers may be at risk of vitamin D deficiency. As the fetus and infant rely on the vitamin D levels in the mother’s blood and breast milk, it is crucial to maintain sufficient reserves of vitamin D.8
The majority of premature infants suffer from vitamin D deficiency. This deficiency is found to be more pronounced in stillborn infants compared to those who survive. Hence, assessing the level of vitamin D in the umbilical cord could potentially aid in predicting the prognosis of premature infants. Furthermore, to enhance the health outcomes of both mothers and newborns, it is recommended that vitamin D supplementation be included as a standard part of prenatal care for all expectant mothers.9
Hence, vitamin D deficiency in expectant mothers can reduce this vital nutrient transfer to the fetus via the placenta. This deficiency can harm both the baby and the mother, potentially influencing the baby’s health.10 Recent research indicates that vitamin D deficiency in premature infants is linked to a host of complications, including infection, cerebral hemorrhage, retinopathy of prematurity, respiratory distress syndrome (RDS), transient tachypnea of the newborn (TTN), and even mortality. Correcting vitamin D levels in newborns could potentially reduce the incidence of some of these significant complications.11–13 Hence, this study aimed to examine the impact of vitamin D intake at birth on neonatal outcomes.
Methods
The current double-blind clinical trial involved 100 infants admitted to the neonatal intensive care unit of Ghaem Hospital in Mashhad, Iran, from 2021 to 2022.
The inclusion critera for this study were premature infants < 34 weeks old, requiring admission to neonatal intensive case unit. Infants with noticeable congenital anomalies, congenital infections, died in the delivery room, as well as infants born to mothers with a history of drug abuse or asthma were excluded from the study [Figure 1].
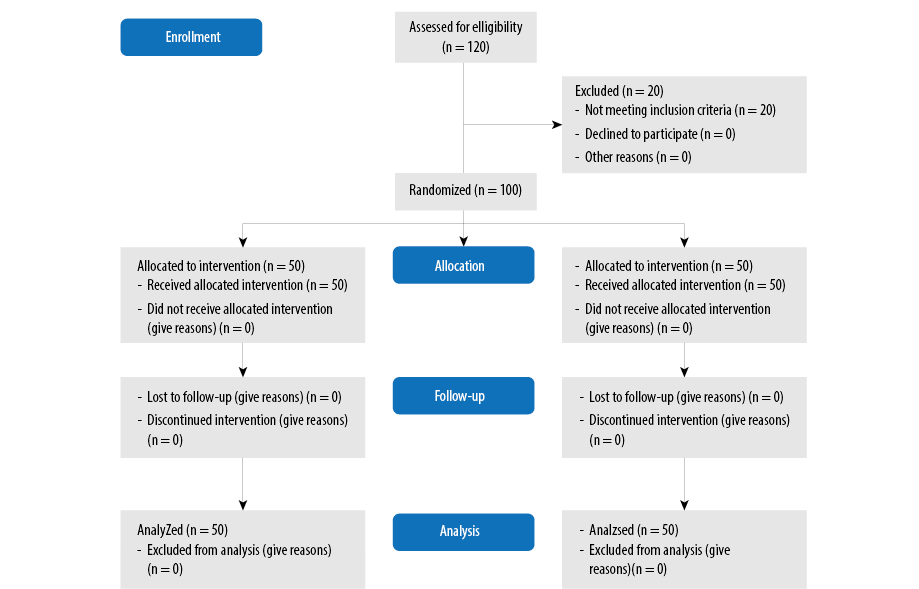 Figure 1: The Consolidated Standards of Reporting Trials flow chart.
Figure 1: The Consolidated Standards of Reporting Trials flow chart.
A sample size of 44 cases was estimated for each group, based on a study by Holick,14 considering the 82% chance of premature infants experiencing respiratory distress that requires oxygen and assuming a 42% difference between the intervention and control groups. This estimation considered an alpha of 0.01 and a beta of 0.1. Ultimately, each group comprised 50 cases, considering a potential attrition rate of 10%.
Using a method of concealment, the participants were randomly divided into the intervention (n = 50) and control (n = 50) groups. This division was achieved through sealed envelopes and a random number table.
On the first day after birth, the intervention group was administered 10 000 units of vitamin D. In contrast, the control group did not receive any vitamin D supplementation at birth. Before the intervention, the newborns’ blood samples were collected for routine tests, and their serum vitamin D levels were measured. The samples were prepared and centrifuged, after which the serum was stored at -20 °C before being sent for laboratory analysis. Vitamin D levels were assessed using an enzyme-linked immunosorbent assay (ELISA) Reader (RT2100c, Germany) and an ELISA Washing machine, employing the ELISA method. Vitamin D deficiency was defined as values < 30 nmol/L, while values > 30 nmol/L were deemed sufficient. Vitamin D deficiency was categorized into three groups: severe (< 10 ng/mL), moderate (10–20 ng/mL), and mild deficiency (20–30 ng/mL).
As prescribed by the attending physician, the standard procedures were followed by routine practices for both groups. Infants were evaluated and compared between the two groups during hospitalization based on various parameters. These included clinical symptoms, oxygen requirements, duration of oxygen therapy, necessity and duration of mechanical ventilation, method of oxygen therapy, and occurrence of infection.
All analyses were conducted using SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.). The descriptive data were presented as mean±SD and percentages. Before the analysis, the normality of the data was verified using the one-sample Kolmogorov-Smirnov test. The data were analyzed using Student’s t-test, Mann-Whitney U, and chi-square tests. A p-value of < 0.05 determined statistical significance.
The study protocol received approval from the Ethics Committees of Mashhad University of Medical Sciences (Approval Number: IR.MUMS.MEDICAL.REC.1399.572). Additionally, the study was registered with the Iranian Registry for Clinical Trials (Registration Code: IRCT20110807007244N6).
Results
The study included 100 infants, comprising 56 (56.0%) boys and 44 (44.0%) girls. Twenty infants were excluded from the present study due to not meeting the inclusion criteria. The intervention and control groups were homogeneous in terms of parity (p = 0.440), gestational age (p = 0.257), betamethasone administration (p = 0.740), and pregnancy complications (p > 0.100) [Table 1]. In both groups, the highest frequency corresponded to one pregnancy (p = 0.440), one delivery (p = 0.470), and the absence of pregnancy complications (p = 0.200). Regarding the type of delivery, the rate of cesarean section in the control group was significantly higher than in the intervention group (p = 0.020).
Table 1: Maternal characteristics in the intervention and control groups.
|
Gestational age
|
|
|
|
|
25–28 weeks
|
6 (12.0)
|
7 (14.0)
|
0.257*
|
|
28–32 weeks
|
24 (48.0)
|
16 (32.0)
|
|
|
32–34 weeks
|
20 (40.0)
|
27 (54.0)
|
|
|
Parity
|
|
|
|
|
One
|
20 (40.0)
|
21 (42.0)
|
0.440*
|
|
Two
|
13 (26.0)
|
8 (16.0)
|
|
|
Three and more
|
17 (34.0)
|
21 (42.0)
|
|
|
Deliveries
|
|
|
|
|
Zero
|
24 (48.0)
|
26 (52.0)
|
0.470*
|
|
One
|
14 (28.0)
|
9 (18.0)
|
|
|
Two and more
|
15 (30.0)
|
12 (24.0)
|
|
|
Prenatal care
|
|
|
|
|
Yes
|
49 (98.0)
|
49 (98.0)
|
0.990*
|
|
No
|
1 (2.0)
|
1 (2.0)
|
|
|
Mode of delivery
|
|
|
|
|
Vaginal
|
18 (36.0)
|
7 (14.0)
|
0.020*
|
|
Cesarean
|
32 (64.0)
|
43 (86.0)
|
|
|
Uses betamethasone
|
|
|
|
|
Yes
|
42 (84.0)
|
44 (88.0)
|
0.740*
|
|
No
|
7 (14.0)
|
6 (12.0)
|
|
|
Pregnancy complications
|
|
|
|
|
None
|
28 (56.0)
|
25 (50.0)
|
0.200*
|
|
Preeclampsia
|
8 (16.0)
|
6 (12.0)
|
|
|
Hypertension
|
4 (8.0)
|
1 (2.0)
|
|
|
Premature rupture of membranes
|
12 (24.0)
|
9 (18.0)
|
|
|
Gestational diabetes mellitus
|
1 (2.0)
|
6 (12.0)
|
|
|
Sex
|
|
|
|
|
Male
|
25 (50.0)
|
31 (62.0)
|
0.220*
|
|
Female
|
25 (50.0)
|
19 (38.0)
|
|
|
Birth weight, g (mean ± SD)
|
1575.3 ± 469.1
|
1627.3 ± 508.0
|
0.598**
|
|
Vitamin D level, ng/mL (mean ± SD)
|
26.7 ± 12.8
|
26.1 ± 12.0
|
0.792**
|
*Chi-square; **T-test
There was no significant difference in the sex distribution of the infants between the two groups (p = 0.220). Furthermore, there were no significant differences between the groups in terms of neonatal prognosis (p = 0.430), average length of hospital stays (p = 0.530), birth weight (p = 0.598), vitamin D levels (p = 0.792), and amniotic fluid volume
(p = 0.630) [Table 2].
Table 2: Infant characteristics in the intervention and control groups.
|
Sex
|
|
|
|
|
Male
|
25 (50.0)
|
31 (62.0)
|
0.220*
|
|
Female
|
25 (50.0)
|
19 (38.0)
|
|
|
Prognosis
|
|
|
|
|
Death during hospitalization
|
7 (14.0)
|
7 (14.0)
|
0.430*
|
|
Hospital discharge
|
25 (50.0)
|
19 (38.0)
|
|
* Chi-square; ** T-test
An examination of clinical symptoms revealed that most infants exhibited respiratory distress, 49 (98.0%) in the intervention group and all 50 (100%) in the control group. There was no significant difference in the incidence of respiratory distress between the two groups (p = 0.500). Table 3 shows the frequency distributions of the primary symptoms of respiratory distress in infants.
Table 3: Clinical symptoms seen in infants in the intervention and control groups.
|
Mechanical ventilation
|
|
|
|
|
Yes
|
10 (20.0)
|
20 (40.0)
|
0.030*
|
|
No
|
40 (80.0)
|
30 (60.0)
|
|
|
Duration of ventilation, hours (mean ± SD)
|
25.7 ± 66.5
|
92.2 ± 58.6
|
0.120**
|
|
Surfactant
|
|
|
|
|
Yes
|
16 (32.0)
|
29 (58.0)
|
0.009*
|
*Chi-square; **T-test
The frequency of mechanical ventilation in both control groups was significantly different (p = 0.030). This suggests that the control group of infants received more mechanical ventilation than the intervention group. However, when it comes to the duration of mechanical ventilation, no significant difference was observed between the two groups
(p = 0.120). No significant difference was observed between the two groups in terms of continuous positive airway pressure (p = 0.500) and its duration (p = 0.270), high-flow nasal cannula (p = 0.090) and its duration (p = 0.140), and oxygen hood (p = 0.550), and its duration (p = 0.540).
A significant difference was observed between the groups regarding surfactant administration (p = 0.009) and the number of times the surfactant was administered (p = 0.020). This indicates that the intake of surfactant in the intervention group was significantly less than in the control group.
Discussion
The administration of 10 000 IU of vitamin D to premature infants reduces respiratory issues, thereby decreasing the need for both initial and advanced respiratory care. Additionally, it decreases the requirement for surfactant administration. To the best of our knowledge, this is the first clinical trial investigating respiratory complications associated with vitamin D administration in neonates. Moreover, the usage frequency of NIV was significantly lower in the intervention group compared to the control group. A recent study conducted by Boskabadi et al. 2019, revealed a significant correlation between the level of vitamin D in infants and the incidence of RDS and the level of vitamin D in the mother.9 The findings of this study align with those of the current study, indicating an increased need for respiratory support in premature infants who have a deficiency in vitamin D. In line with our study, research conducted in China demonstrated that early intake of vitamin D can significantly decrease the incidence of bronchopulmonary dysplasia in premature infants. Furthermore, this early vitamin D consumption can significantly reduce the serum level of 25-hydroxyvitamin D3. As a result, it prevents bronchopulmonary dysplasia in infants.15
Onwuneme et al,16 demonstrated that preterm infants with low levels of 25-hydroxyvitamin D3 (less than 30 nmol/L) at birth were associated with an increased need for oxygen, a longer duration of intermittent positive pressure ventilation during labor resuscitation, and a greater requirement for assisted ventilation.
Contrary to this study, Fallahi et al,17 found that when comparing groups with vitamin D deficiency and normal levels, there was no difference in the duration of mechanical ventilation between the groups. In other words, both groups had similar durations of mechanical ventilation.17
Embryos receive vitamin D solely from their mothers. The amount transferred depends on the mother’s exposure to sunlight and vitamin D consumption.18 During pregnancy, levels of 25-hydroxyvitamin D3 increase during the first trimester and peak in the third trimester, which is twice the levels observed outside of pregnancy. Levels of vitamin D-binding protein rise in correlation with an increase in the mother’s serum vitamin D levels.19 Having missed this crucial period, premature neonates are at risk of vitamin D deficiency. This is because essential nutrients, including vitamin D, are primarily transmitted during the third trimester of pregnancy.20 According to Boskabadi et al,9 nearly half of all premature infants suffer from severe vitamin D deficiencies. Preterm neonates with vitamin D deficiency were found to be deficient in 89% of cases, respectively, and half of them were severely deficient.21
The emerging field of research that focuses on the impact of vitamin D on lung growth and maturation in early life, as well as its influence on lung diseases and respiratory symptoms, is intriguing. Numerous studies suggest that a deficiency in vitamin D may pose a risk factor for RDS in infants born prematurely. In a similar study, Ataseven et al,22 discovered that premature infants with severe vitamin D deficiency are more likely to experience RDS than those with mild to moderate vitamin D deficiency. Mohamed Hegazy et al,23 demonstrated that the average concentration of 25-hydroxyvitamin D3 in the serum of premature infants suffering from RDS is significantly lower compared to those without the syndrome. Moreover, patients suffering from RDS who also have low levels of 25-hydroxyvitamin D3 tend to have longer hospital stays compared to those without the syndrome. Similarly, Yu et al,24 suggested that a deficiency in vitamin D could potentially be linked to an increased risk of RDS in infants born prematurely. They also discovered that maintaining adequate levels of vitamin D could potentially be beneficial for the maturation of lungs in humans.
Alveolar epithelial-mesenchymal interactions are crucial in perinatal lung development.25 Premature birth, caused by structural lung immaturity and inadequate surfactant production, is associated with RDS in humans.26 In an observational study, Koroglu and colleagues observed increased mechanical ventilation and surfactant treatment among 109 preterm infants deficient in vitamin D.27 Vitamin D reduces lung injury by stimulating the proliferation and migration of ATII cells, reducing epithelial cell apoptosis, and inhibiting TGF-β-induced epithelial-mesenchymal transition (EMT). Vitamin D treatment has also been reported to significantly reduce inflammatory responses and cellular apoptosis in the lung tissue of mice model asthma.28 Another study reported that vitamin D has therapeutic potential for acute RDS.29
A case-control study on 160 preterm infants, conducted in Mashhad, Iran, assessing the relationship between vitamin D levels and respiratory distress revealed that infants with respiratory distress had significantly lower maternal and neonatal vitamin D levels than those without respiratory distress.30 Factors such as duration of hospitalization, gestational age, birth weight, Apgar scores, and head circumference were associated with neonatal vitamin D levels. Complications, including death and pneumothorax, were observed among infants with respiratory distress. These findings highlight the potential protective role of adequate vitamin D levels against respiratory distress in preterm infants, underscoring the importance of further research and interventions to optimize neonatal health outcomes.
Another case-control study investigated the association between serum vitamin D levels and TTN, a common cause of respiratory distress in neonates.31 The research, carried out from 2016 to 2019 in a general hospital affiliated with Mashhad University of Medical Sciences, Iran, included 34 infants with TTN and 82 neonates in the control group, along with their mothers. The study found that infants with TTN and their mothers had significantly lower serum vitamin D levels than the control group. Specifically, the mean differences in neonatal and maternal vitamin D levels between the two groups were 11.10 ng/mL and 13.36 ng/mL, respectively. Additionally, all infants in the TTN group had vitamin D levels below 30 ng/mL, with the majority exhibiting severe deficiency, highlighting the potential importance of addressing maternal vitamin D deficiency in reducing the incidence of TTN in newborns.
Moreover, Pourbadakhshan et al,32 conducted a single-blind clinical trial to assess the impact of administering 50 000 units of 25-hydroxy vitamin D to pregnant women at risk of preterm delivery on the incidence of non-specific RDS in their newborns.The study included mothers and neonates with a gestational age of 32–37 weeks. Results showed a significant difference between the intervention and control groups regarding infant weight, one-minute and five-minute Apgar scores, and the incidence of non-specific RDS. However, there were no significant differences between the groups regarding gender, type of delivery, and levels of 25-hydroxyvitamin D3 in the mother and infant. The findings suggest that a single vitamin D injection in pregnant women at risk of preterm birth reduces transient respiratory problems in their newborns.
One limitation of this study is the lack of vitamin D level testing after injection. In addition, premature babies are more susceptible to different problems simultaneously, which affects the accurate identification of patients with respiratory problems. More studies should be conducted on a larger population of preterm infants to evaluate the relationship between maternal and infant vitamin D status. Future studies should examine the adaptation of newborns to mothers’ vitamin D levels.
Conclusion
Vitamin D consumption at birth is effective in the prognosis of premature infants. It reduces the need for NIV, mechanical ventilation, and surfactant. Generally, vitamin D consumption at birth reduces the incidence of respiratory complications in premature infants.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Attar SM, Siddiqui AM. Vitamin d deficiency in patients with systemic lupus erythematosus. Oman Med J 2013 Jan;28(1):42-47.
- 2. Fitri A, Sjahrir H, Bachtiar A, Ichwan M. Modulation of interleukin-8 production by vitamin D supplementation in Indonesian patients with diabetic polyneuropathy: a randomized clinical trial. Oman Med J 2020 Sep;35(5):e168.
- 3. Gordon SM, Srinivasan L, Harris MC. Neonatal meningitis: overcoming challenges in diagnosis, prognosis, and treatment with omics. Front Pediatr 2017 Jun;5:139.
- 4. Waitzman NJ, Jalali A, Grosse SD, editors. Preterm birth lifetime costs in the United States in 2016: an update. Seminars in perinatology. Elsevier; 2021.
- 5. da Fonseca EB, Damião R, Moreira DA. Preterm birth prevention. Best Pract Res Clin Obstet Gynaecol 2020 Nov;69:40-49.
- 6. Christoph P, Challande P, Raio L, Surbek D. High prevalence of severe vitamin D deficiency during the first trimester in pregnant women in Switzerland and its potential contributions to adverse outcomes in the pregnancy. Swiss Med Wkly 2020 May;150:w20238.
- 7. Singleton R, Day G, Thomas T, Schroth R, Klejka J, Lenaker D, et al. Association of maternal vitamin D deficiency with early childhood caries. J Dent Res 2019 May;98(5):549-555.
- 8. Voltas N, Canals J, Hernández-Martínez C, Serrat N, Basora J, Arija V. Effect of vitamin d status during pregnancy on infant neurodevelopment: the ECLIPSES study. Nutrients 2020 Oct;12(10):3196.
- 9. Boskabadi H, Maamouri G, Hemmatipour A, Parvini Z, Ramazani A, Bagheri F. Comparison of serum vitamin D in the umbilical cord of survived with not survived premature infants. Iran J Pediatr 2019;29(3).
- 10. EMARA MM. Correlation between vitamin D deficiency in pregnancy and low birth weight neonates. Med J Cairo Univ 2022;90(9):1597-1602.
- 11. Boskabadi H, Zakerihamidi M, Faramarzi R. The vitamin D level in umbilical cord blood in premature infants with or without intra-ventricular hemorrhage: a cross-sectional study. Int J Reprod Biomed 2018 Jul;16(7):429-434.
- 12. Boskabadi H, Heidari E. Correlation of vitamin D serum level of mothers and their newborns. Modern Care Journal 2021;18(2).
- 13. Boskabadi H, Rakhshanizadeh F, Maryam Z. Comparison of vitamin D levels between mothers and infants with and without prolonged membrane rupture. Infektsiia Immun 2022;12(3):556-562.
- 14. Holick MF. Vitamin D deficiency. N Engl J Med 2007 Jul;357(3):266-281.
- 15. Ge H, Qiao Y, Ge J, Li J, Hu K, Chen X, et al. Effects of early vitamin D supplementation on the prevention of bronchopulmonary dysplasia in preterm infants. Pediatr Pulmonol 2022 Apr;57(4):1015-1021.
- 16. Onwuneme C, Martin F, McCarthy R, Carroll A, Segurado R, Murphy J, et al. The association of vitamin D status with acute respiratory morbidity in preterm infants. J Pediatr 2015;166(5):1175-1180.e1.
- 17. Fallahi M, Afjeh A, Saneifard H, Namazi N, Kazemian M, Tabatabaee S. Comparison of vitamin D level in preterm and term infant–mother pairs: a brief study. Iranian Journal of Neonatology IJN 2016;7(1):32-36.
- 18. Taylor SN. Vitamin D in toddlers, preschool children, and adolescents. Ann Nutr Metab 2020;76(Suppl 2):30-41.
- 19. Ko D-H, Jun S-H, Nam Y, Song SH, Han M, Yun Y-M, et al. Multiplex LC-MS/MS for simultaneous determination of 25-hydroxyvitamin D, 24,25-dihydroxyvitamin D3, albumin, and vitamin D-binding protein with its isoforms: one-step estimation of bioavailable vitamin D and vitamin D metabolite ratio. J Steroid Biochem Mol Biol 2021 Feb;206:105796.
- 20. Abrams SA. Vitamin D in preterm and full-term infants. Ann Nutr Metab 2020;76(Suppl 2):6-14.
- 21. Park S-H, Lee G-M, Moon J-E, Kim H-M. Severe vitamin D deficiency in preterm infants: maternal and neonatal clinical features. Korean J Pediatr 2015 Nov;58(11):427-433.
- 22. Ataseven F, Aygün C, Okuyucu A, Bedir A, Kücük Y, Kücüködük S. Is vitamin d deficiency a risk factor for respiratory distress syndrome? Int J Vitam Nutr Res 2013;83(4):232-237.
- 23. Mohamed Hegazy A, Mohamed Shinkar D, Refaat Mohamed N, Abdalla Gaber H. Association between serum 25 (OH) vitamin D level at birth and respiratory morbidities among preterm neonates. J Matern Fetal Neonatal Med 2018 Oct;31(20):2649-2655.
- 24. Yu R-Q, Chen D-Z, Hao X-Q, Jiang S-H, Fang G-D, Zhou Q. Relationship between serum 25 (OH) D levels at birth and respiratory distress syndrome in preterm infants. Zhongguo Dang dai er ke za zhi= Chinese Journal of Contemporary Pediatrics 2017;19(11):1134-1137.
- 25. Torday JS, Rehan VK. Developmental cell/molecular biologic approach to the etiology and treatment of bronchopulmonary dysplasia. Pediatr Res 2007 Jul;62(1):2-7.
- 26. Smart DE, Princivalle MB. Improving RDS treatment with current drugs. J Matern Fetal Neonatal Med 2012 Aug;25(8):1209-1211.
- 27. Koroglu OA, Onay H, Cakmak B, Bilgin B, Yalaz M, Tunc S, et al. Association of vitamin D receptor gene polymorphisms and bronchopulmonary dysplasia. Pediatr Res 2014 Aug;76(2):171-176.
- 28. Zhang H, Yang N, Wang T, Dai B, Shang Y. Vitamin D reduces inflammatory response in asthmatic mice through HMGB1/TLR4/NFκB signaling pathway. Mol Med Rep 2018 Feb;17(2):2915-2920.
- 29. Zheng S, Yang J, Hu X, Li M, Wang Q, Dancer RC, et al. Vitamin D attenuates lung injury via stimulating epithelial repair, reducing epithelial cell apoptosis and inhibits TGF-β induced epithelial to mesenchymal transition. Biochem Pharmacol 2020 Jul;177:113955.
- 30. Boskabadi H, Mamoori G, Khatami SF, Faramarzi R. Serum level of vitamin D in preterm infants and its association with premature-related respiratory complications: a case-control study. Electron Physician 2018 Jan;10(1):6208-6214.
- 31. Boskabadi H, Maamouri G, Kalani-Moghaddam F, Ataee Nakhaei MH, Zakerihamidi M, Rakhshanizadeh F. Comparison of umbilical cord serum vitamin D levels between infants with transient tachypnea of the newborn and those without respiratory distress. Arch Iran Med 2020 Aug;23(8):530-535.
- 32. Pourbadakhshan N, Boskabadi H, Nakhaei MH, Darabi A, Sani MR. The effect of maternal vitamin D intake on the incidence of nonspecific respiratory distress in infants: a randomized clinical trial. Clin Exp Obstet Gynecol 2023;50(4):78.