Cognition is the capability to analyze, comprehend, solve problems, memorize, and remember information.1 Cognitive impairment can significantly impede an individual’s daily activities and social life, in addition to burdening their families and healthcare providers.2
The levels of cognitive impairment may range from mild cognitive impairment (MCI) to very severe cognitive decline such as in stage 7 dementia.3 In 2015, approximately 47 million people worldwide have dementia, representing a financial burden of USD 818 billion, estimated to triple by 2050. Meanwhile, 85% of those costs are directly associated with family and social support instead of medical care.4 Several studies suggest that by 70 years of age, 16% of the world population will have MCI, and 14% will suffer from dementia.5 As the world population ages, these disease burdens are likely to get worse.1 Therefore, the current health policy options seem limited to strategic public health promotion and prevention.
As 35% of hypertension cases (and thereby the risk for cognitive impairment) are attributable to a combination of modifiable lifestyle-related risk factors, an active healthy and prosocial lifestyle has been recommended for individuals aged ≥ 45 years. These include physical activity, cessation of smoking, and engaging in social activities. These strategies are believed to prevent or delay a third of cognitive impairment cases.4
Numerous studies have shown a consistent association between smoking and cognitive impairment. However, there is a lack of studies regarding passive smoking and cognitive impairment, as cigarette smoke exposure contributes to complex pro-inflammatory responses in neuronal cells.6 Passive smoking, also known as secondhand smoke, refers to inhalation of others’ tobacco smoke. Passive smoking poses a health risk for all age groups, and children are most at risk.7 As the world population ages, the prevalence of cognitive impairment is expected to rise significantly in the future. This will be especially burdensome for low-to-middle-income economies such as Indonesia.
According to recent estimates, 40.3% of Indonesia’s population are active smokers. Moreover, 37.7% of adolescents aged 13–15 years have smoked cigarettes.8 More than 70% of Indonesian active smokers indulge in the habit 2–3 times daily inside their houses in the proximity of their non-smoking family members.9 These statistics portent an alarming rise in the country’s healthcare burden.10 Given these issues, this study aimed to determine the association between smoking, passive smoking, and cognitive impairment in Indonesia.
Methods
This population-based epidemiologic study was performed from 2021 to 2023 in four rural townships in the Central Java. Java was selected because it is the most densely populated and centrally-located Indonesian island. Through a simple random sampling method, the four specific sites—Giritontro, Pracimantoro, Pedan, and Karangdowo—were selected as they were assumed to be reasonably representative of the Indonesian population. We focused on the 180 000 adult population of this region. Each town has a community health center, catering to an estimated population of 30 000. The study was approved by the Health Research Ethics Committee of Sebelas Maret University (No: 71/UN27.06.11/KEP/EC/2021, Date: 31 March 2021).
The participants’ medical records were retrieved from the community health information system (Sistem Informasi Manajemen Puskesmas). In the first attempt at participant recruitment, we obtained 17 526 families that met our inclusion criteria, defined as having at least one active smoking person in a family and at least one non-smoking adult member living in the same house. Using a simple random sampling method, we shortlisted 3179 potential participants aged ≥ 30 years from whom informed participation consent was obtained. Details of the cognitive impairment symptoms, lifestyle, psychosocial characteristics, anthropometrics, and comorbidities were obtained from their responses to a physician-administered questionnaire. Excluded from the study were individuals with cognitive impairment due to degenerative processes, metabolic disturbances such as thyroid diseases, Cushing syndrome, pernicious anemia, substance abuse, alcohol consumption or abuse, or depressive disorders.11
Cognitive impairment was assessed using Mini-Mental State Examination (MMSE), a validated global assessment scale of cognitive status that takes only seven minutes to administer.12,13 The MMSE assesses orientation to place and time (10 points), concentration (five points), short-term memory or recall (three points), language (fluency, comprehension, repetition), and visuospatial abilities (nine points). The normal cognitive score of MMSE ranges 30–24. The severity of cognitive impairment is classified into three categories as MCI (MMSE score of 23–20), moderate dementia (19–10), and severe dementia (9–0).12
The MMSE was administered by two qualified physicians. During visits to public health centers in the four designated areas, the active smoking status of individuals was obtained through direct anamnesis or auto anamnesis. This information was systematically recorded using computerized data entry, ensuring detailed and accurate documentation of each participant’s smoking status. The data collection process included capturing comprehensive details such as the frequency and duration of smoking, types of tobacco products used, and any cessation attempts. This method aimed to provide reliable data for further analysis of smoking behaviors within these communities.
The status of active smoking was obtained from the participants by self-report, with the baseline assessment of whether they were smoking currently and had smoked at least 100 tobacco cigarettes in their lifetime. Its status was classified into never (< 100 and currently not smoking), former (> 100 and currently not smoking), and current smoker (> 100 and currently smoking). For this, we adopted the criteria by Kenkel et al,14 where retrospective self-reporting information on smoking behavior applies to prevalence rates with retrospective data sources adjusted to current levels. The pack-years smoked were calculated by multiplying units of 20 cigarettes or one pack per day with the number of smoking years. Pack years were classified as ≥ 20, 19–10, and < 10 years. Such retrospective categorization has previously been shown to minimize misclassification bias.15
Passive smoking was interpreted as a non-smoking person living with active smokers who smoked daily at home. Tobacco smoke exposure due to passive smoking at home was assessed by self-report. The variables of smoking status, years of smoking, and exposure to passive smoking were classified into five groups to avoid the effects of passive and active smoking and evaluate dose-response patterns.
The participants were thus classified as ‘never smoking’, ‘no exposure to passive smoke’, ‘active smokers without exposure to passive smoking’, and ‘current or former smokers with ≥ 20, 19–10, and < 10 pack-years’.
The categorical variables comprised: age group (30–39, 40–49, 50–59, and ≥ 60 years); sex (male or female); education (illiterate, primary, secondary, and higher); body mass index (BMI) (< 25, 25–30, and ≥ 30 kg/m2); physical activity according to Physical Activity Scale for the Elderly (PASE) scores (< 100, 100–249, and ≥ 250), higher scores representing greater physical activity;16 and comorbidities (cardiovascular disease (CVD), hypertension, and diabetes mellitus (DM)).
Continuous variables were analyzed using means and SD. Descriptive statistics were used for categorical variables, using simple proportions for sample analyses. The association between cognitive impairment and both active and passive smoking was determined using linear regression. Multiple linear regression models were used to adjust for potentially confounding covariates. We also reported the adjusted mean and regression coefficients of MMSE scores.
The analyses were reperformed with cognitive impairment redefined as a dichotomous variable, with an MMSE score below 24 indicating dementia. The relationship between dichotomous cognitive impairment and smoking status was evaluated using logistic regression analysis. The strength of their relationship was assessed using odds ratio (OR). The reliability of OR was tested using 95% CI. Multiple logistic regression models were used to adjust for potential confounders.
Multiple imputations were used to account for missing data.17 Indonesia’s population (as per the 2020 census) determined the post-stratification of weights. All statistical analyses were performed by SPSS Statistics (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.).
Results
The study participants comprised 409 adults (264 male and 145 female). Their demographic data is listed in Table 1. Nearly half of the participants (48.2%) were illiterate. The majority (317; 75.5%) were active smokers and 158 (38.6%) were heavy smokers (≥ 20 pack-year). Passive smokers numbered 271 (66.3%). Moderate dementia constituted the most prevalent cognitive impairment (44.0%). Nearly half (45.5%) of participants had a low BMI (< 25 kg/m2) and 56.7% had PASE scores below 100. Moreover, some participants had multiple comorbidities (CVD, hypertension, or DM).
Table 1: The demographic profiles of the study participants (N = 409).
|
Sex
|
|
Male
|
264
|
64.5
|
|
Female
|
145
|
35.5
|
|
Education
|
|
Illiterate
|
197
|
48.2
|
|
Primary
|
109
|
26.7
|
|
Secondary
|
77
|
18.8
|
|
Higher
|
25
|
6.1
|
|
Cognitive impairment (MMSE)
|
|
Normal
|
116
|
28.4
|
|
MCI
|
97
|
23.7
|
|
Moderate dementia
|
180
|
44.0
|
|
Dementia
|
16
|
3.9
|
|
Smoking
|
|
Never
|
101
|
24.7
|
|
Former
|
150
|
36.7
|
|
Present
|
158
|
38.6
|
|
Pack years
|
|
None
|
91
|
22.2
|
|
< 10
|
67
|
16.4
|
|
10–19
|
92
|
22.5
|
|
≥ 20
|
158
|
38.6
|
|
Passive smoking
|
|
No
|
138
|
33.7
|
|
Yes
|
271
|
66.3
|
|
Body mass index, kg/m2
|
|
|
|
< 25
|
186
|
45.5
|
|
25–30
|
121
|
29.6
|
|
≥ 30
|
102
|
24.9
|
|
Physical activity (PASE score)
|
|
< 100
|
232
|
56.7
|
|
100–249
|
118
|
28.9
|
|
≥ 250
|
59
|
14.4
|
|
Comorbidities
|
|
Cardiovascular disease
|
175
|
42.8
|
|
Hypertension
|
239
|
58.4
|
MMSE: Mini-Mental State Examination; MCI: mild cognitive impairment; PASE: Physical Activity Scale for the Elderly.
The results were shown for all participants and were not stratified by sex, as the relationship between cognitive impairment and smoking was found to be similar for men and women. MCI constituted the most prevalent cognitive impairment type in the 40–49 age group. The prevalence of moderate dementia and dementia increased with age [Figure 1].
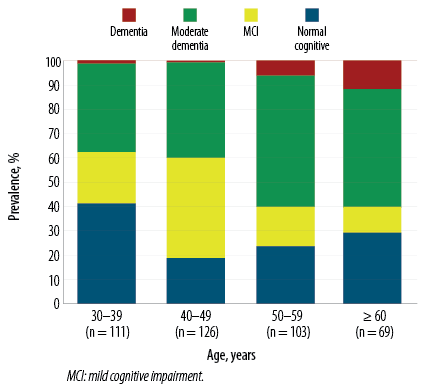 Figure 1: Prevalence of cognitive impairment stratified by age groups.
Figure 1: Prevalence of cognitive impairment stratified by age groups.
The age-adjusted effect of passive smoking status on cognitive impairment by three active smoking statuses such as never, former, and current is presented in Figure 2. Each comparison within three active smoking categories showed a significant difference (p < 0.050) in the MMSE score. They also showed that the age-adjusted mean was consistently lower among passive smokers. However, the impact of leaving smoking could not be evaluated directly because the smoking cessation dates were not gathered. Significant differences between current and former active smokers were noticed, while mean MMSE scores declined as pack years increased [Table 2].
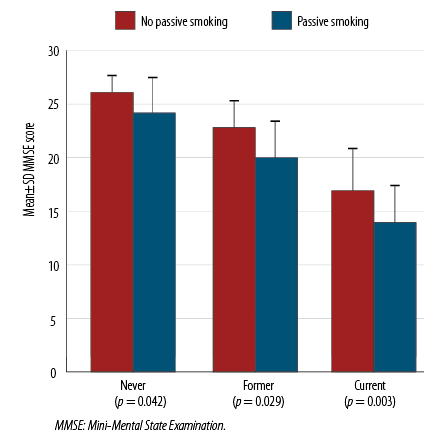 Figure 2: The effect of passive smoking status on cognitive impairment by three active smoking
Figure 2: The effect of passive smoking status on cognitive impairment by three active smoking
status categories.
Table 2: The relationship between smoking and cognitive impairment presents a dose-response trend with increased pack years.
|
Constant
|
22.27
|
|
23.09
|
|
22.03
|
|
|
Never smoking/no passive smoking
|
Reference
|
|
Reference
|
|
Reference
|
|
|
Never smoking/passive smoking
|
-0.71
|
-1.91–0.95
|
-0.61
|
-1.98–0.93
|
-0.38
|
-1.68–1.02
|
|
Pack years
|
|
|
|
|
|
|
|
< 10
|
-0.42
|
-0.98–0.62
|
-0.23
|
-1.06–0.66
|
0.09
|
-0.79–0.92
|
|
10-19
|
-0.92
|
-2.01–0.26
|
-0.53
|
-1.75–0.92
|
-0.72
|
-1.86–0.68
|
|
≥ 20
|
-4.25
|
-5.32–-2.96
|
-2.69
|
-3.28–-1.06
|
-2.08
|
-2.36–-0.45
|
|
Overall p-value of F-test
|
< 0.001
|
|
0.029
|
|
0.041
|
|
|
Trend test p-valuec
|
< 0.001
|
|
0.002
|
|
0.038
|
|
|
Never smoking/no passive smoking
|
1.00
|
|
1.00
|
|
1.00
|
|
|
Never smoking/passive smoking
|
2.19
|
1.43–2.89
|
2.08
|
1.43–2.88
|
2.01
|
1.37–2.70
|
|
Pack years
|
|
|
|
|
|
|
|
< 10
|
1.93
|
1.52–2.41
|
1.78
|
1.29–2.32
|
1.53
|
1.02–2.11
|
|
10–19
|
2.05
|
1.38–2.70
|
1.65
|
1.01–2.25
|
1.86
|
1.24–2.42
|
|
≥ 20
|
4.01
|
2.94–5.10
|
1.86
|
0.98–2.73
|
1.61
|
0.98–2.31
|
|
Overall p-value of F-test
|
< 0.001
|
|
0.072
|
|
0.098
|
|
β: regression coefficient; MMSE: Mini-Mental State Examination; MCI: Mild Cognitive Impairment OR: odd ratio. aA djusted for sex, education, physical activity based on Physical Activity Scale for the Elderly score, comorbidities such as cardiovascular disease, hypertension, and diabetes mellitus; blinear regression coefficient; cthe p-value of linear trend test covering pack-year variable accompanied by variable of never smoking/no passive smoking group as category of reference.
The relationship between cognitive impairment (evaluated using the continuous MMSE) and smoking (using six categorical variables integrating passive and active smoking status and pack years information) is shown in Table 2. It indicates that elevated pack-years smoking was accompanied by lower MMSE scores or higher severity of cognitive impairment. This effect is particularly pronounced among those with a smoking exposure of ≥ 20 pack years. Furthermore, its effect persisted significantly (coefficient of regression excludes 0 with 95% CI) after adjusting for factors such as sex, education level, PASE, and comorbidities (CVD, hypertension, and DM).
A declining trend in MMSE score was observed with an increase in smoking pack years. This trend remained significant after adjusting for possible confounding factors (p-value = 0.038). In participants who never actively smoked, a few significant passive smoking effects were observed. Multivariate-adjusted regression coefficient (β = -0.38; 95% CI: -1.68–-1.02) was similar to that of 10–19 pack-year smokers (β = -0.72; 95% CI: -1.86–0.68). Repeat analysis returned similar results, and cognitive impairment was interpreted as a dichotomous variable with an MMSE score cutoff of ≤ 23. Increased pack-years followed an increased OR that caused a dose-response effect (p-value = 0.023) and (OR= 1.161; 95% CI: 0.98–2.31) for those exposed to ≥ 20 pack years. The extent of the relationship for passive smoking across never smoking with (OR = 2.01; 95% CI: 1.37–2.70) was proportional to that of 10–19 pack year active smokers with OR of 1.86 (95% CI: 1.24–2.42), suggesting that passive smoking can have a significant impact on cognitive health.
Discussion
Regarding active smoking and cognitive impairment, our results were similar to those from other countries. For example, a study on American senior citizens has shown that tobacco smoking is related to the risk of cognitive dysfunction. This includes former smokers who have smoked > 100 cigarettes in their lifetime and then quit (OR= 3.12; 95% CI: 1.51–4.73).18 A case-control study in Poland which investigated the association between tobacco smoking and cognitive performance in patients with psychosis found that smokers with schizophrenia had lower scores on delayed memory tests and immediate memory compared to non-smokers with schizophrenia (47.1±6.4; p = 0.002 vs. 52.0±4.0; p = 0.001, respectively).19 A study among Pakistanis aged 18–30 years showed that non-smokers outperformed smokers in attention-switching tasks (96.95±2.18 vs. 83.75±11.22; p = 0.001).20 Similarly, tobacco smokers aged 18–29 years in the UK showed a significant cognitive decline in sustained attention (p = 0.005), executive planning (p = 0.002), and spatial working memory (p = 0.004) compared to non-smokers even after adjusting for education, income, and gender covariates.21
However, we could not find previous data regarding the effect of passive smoking on cognitive decline.
The present study has revealed a dose-response relationship between smoking (20 pack-year threshold) and cognitive impairment. This relationship persisted even after adjusting for confounding risk factors such as older age, CVD, hypertension, diabetes, and high BMI. Severe smoking was associated with cognitive decline in young-to-middle-aged individuals.
High concentrations of nicotinic acetylcholine receptors, nicotinic α4β2, and α7 receptors in the brain might be caused by nicotine-containing tobacco smoking.22 These receptors cause changes in the hemostasis of dopamine, serotonin, and norepinephrine. As a downstream consequence, high levels of α4β2, α7 receptors, and nicotinic acetylcholine receptors are known to significantly impact vital cognitive domains such as learning, memory, attention, as well as executive and sensorimotor functions.23
Although the pathomechanism of passive smoking on cognition has not been extensively reported, several pathomechanisms in other body systems have been reported. Long-term passive smoking increases the concentration of inflammatory cytokines tumor necrosis factor-α, interleukin (IL)-1β, IL-6, and IL-17A in the lungs and impairs adaptive immunity.24
In general, tobacco smoking deteriorates adaptive immune cells of CD4+ and CD25+ regulatory T cells, T helper cells (Th1/Th2/Th17), B cells and memory T/B lymphocytes, and CD8+ T cells. It also impacts dendritic cells, natural killer cells, and macrophages of innate immunity,25,26 causing an increase in the neurodegenerative components such as quinolinic acid and 3-hydroxykynurenine and a decrease in kynurenic acid which protects the kynurenine pathway. These processes are believed to cause pathological neural changes.27 Tobacco smoking also damages the cortical neurons and causes neuronal necrosis. Consequently, chronic active smoking may lead to brain structural changes, especially in the insula and superior frontal gyrus, compared to non-smokers (p = 0.020 and p = 0.050, respectively) as revealed by resting-state functional magnetic resonance imaging and global brain connectivity method.28 Damage to these brain centers is known to cause amnesia or forgetfulness.26
Our findings aligned with other studies where males smoked more than females. In the US, approximately 16.7% of adult men and 13.6% of women smoked tobacco in 2015.29 In 2021, about 29% of the Indonesian population aged ≥ 15 years were smokers. The rate of female smokers in Indonesia declined from 6.9% in 2000 to 3.7% in 2020. Meanwhile, the 2014 Global Youth Tobacco Survey revealed that 20.3% of Indonesian students aged 13–15 years were active smokers, including 19.4% smoking tobacco, 18.3% smoking cigarettes, and 2.1% consuming smokeless tobacco.9,30
In this study, participants with lower education levels, lower PASE scores, and higher BMI, and active and passive smokers with comorbidities, tended to have more cognitive impairment. A significant impact of lower socioeconomic and education levels on risk for cognitive impairment was reported across 51 studies worldwide. This demography is probably less aware of the long-term consequences of cigarette or tobacco smoking.31 Thus, Indonesian authorities should carry out several capacity-building initiatives for nationwide smoke-free taskforces, for example, human-centered design approaches. These initiatives will create a cleaner and better environment, opening the door to a healthier Indonesia. Obesity and physical inactivity accompanied by tobacco consumption, hypertension, and DM cause lectin-like oxidized-low-density lipoprotein receptor-1 (LOX-1) upregulation, leading to neuronal damage and apoptosis. The expression of LOX-1 also stimulates tumor necrosis factor-α, IL-1, and IL-10.32 Furthermore, LOX-1 causes oxidized low-density lipoprotein degradation, increasing the risk of cerebral and coronary plaque formation.33
This study has some limitations. First, quantifying dose association was formulated and adapted based on previous studies regarding smoking, secondhand smoke, and erectile dysfunction.34 However, due to the huge study population coverage, the dose association was quantified solely from MMSE questionnaires. Second, cognitive impairment can be influenced by numerous confounding factors beyond the scope of this study. Third, our participants had unexpectedly low literacy data compared to Indonesian Statistical Agency data,35 which raises caution regarding the generalizability of the study. The authors recommend further similar studies to evaluate more confounding factors.
Conclusion
This study revealed the correlation between tobacco smoking—both active and passive—and cognitive impairment, and emphasized the dose-response association between cumulative pack years and the risk of cognitive impairment. Our findings corroborate with previous studies from different parts of the world. Moreover, this study has also revealed the alarmingly high prevalence of active and passive smoking and the associated cognitive decline in rural Indonesia. This highlights the urgent need for cessation of tobacco smoking from a young age, especially in the presence of non-smokers in the house. We also recommend that the health authorities initiate a public education campaign focusing on the rural areas, to inculcate popular awareness of the dangers of both active and passive smoking.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgments
We are grateful to Dr. Aisyah F. Putri and Dr. Beata Nino for assisting the data entry process; and neurologist colleagues at Dr. Moewardi and Universitas Sebelas Maret Hospital for providing technical support of manuscript feedback and proofreading. The research dataset is available on reasonable request.
references
- 1. Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med 2014 Aug;30(3):421-442.
- 2. Seidel D, Thyrian JR. Burden of caring for people with dementia - comparing family caregivers and professional caregivers. A descriptive study. J Multidiscip Healthc 2019 Aug;12:655-663.
- 3. Chen Y-X, Liang N, Li X-L, Yang S-H, Wang Y-P, Shi N-N. Diagnosis and treatment for mild cognitive impairment: a systematic review of clinical practice guidelines and consensus statements. Front Neurol 2021 Oct;12:719849.
- 4. Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet 2017 Dec;390(10113):2673-2734.
- 5. Morley JE, Morris JC, Berg-Weger M, Borson S, Carpenter BD, Del Campo N, et al. Brain health: the importance of recognizing cognitive impairment: an IAGG consensus conference. J Am Med Dir Assoc 2015 Sep;16(9):731-739.
- 6. Mazzone P, Tierney W, Hossain M, Puvenna V, Janigro D, Cucullo L. Pathophysiological impact of cigarette smoke exposure on the cerebrovascular system with a focus on the blood-brain barrier: expanding the awareness of smoking toxicity in an underappreciated area. Int J Environ Res Public Health 2010 Dec;7(12):4111-4126.
- 7. Razzak HA, Harbi A, Ahli S. Tobacco smoking prevalence, health risk, and cessation in the UAE. Oman Med J 2020 Jul;35(4):e165.
- 8. Holipah H, Sulistomo HW, Maharani A. Tobacco smoking and risk of all-cause mortality in Indonesia. PLoS One 2020 Dec;15(12):e0242558.
- 9. Fithria F, Adlim M, Jannah SR, Tahlil T. Indonesian adolescents’ perspectives on smoking habits: a qualitative study. BMC Public Health 2021 Jan;21(1):82.
- 10. Nurhasana R, Ratih SP, Djaja K, Hartono RK, Dartanto T. Passive smokers’ support for stronger tobacco control in Indonesia. Int J Environ Res Public Health 2020 Mar;17(6):1942.
- 11. Ghazali S, Aziz AA, Amin RM. Healthy aging and its determinants among community-dwelling older persons in East Coast, Malaysia: a multidimensional assessment. Oman Med J 2023 Nov;38(6):e573.
- 12. Baek MJ, Kim K, Park YH, Kim S. The validity and reliability of the mini-mental state examination-2 for detecting mild cognitive impairment and Alzheimer’s disease in a Korean population. PLoS One 2016 Sep;11(9):e0163792.
- 13. Creavin ST, Wisniewski S, Noel-Storr AH, Trevelyan CM, Hampton T, Rayment D, et al. Mini-mental state examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst Rev 2016 Jan;2016(1):CD011145.
- 14. Kenkel D, Lillard DR, Mathios A. Smoke or fog? The usefulness of retrospectively reported information about smoking. Addiction 2003 Sep;98(9):1307-1313.
- 15. Bernaards CM, Twisk JW, Snel J, Van Mechelen W, Kemper HC. Is calculating pack-years retrospectively a valid method to estimate life-time tobacco smoking? A comparison between prospectively calculated pack-years and retrospectively calculated pack-years. Addiction 2001 Nov;96(11):1653-1661.
- 16. Logan SL, Gottlieb BH, Maitland SB, Meegan D, Spriet LL. The physical activity scale for the elderly (PASE) questionnaire; does it predict physical health? Int J Environ Res Public Health 2013 Aug;10(9):3967-3986.
- 17. Sinha SK, Laird NM, Fitzmaurice GM. Multivariate logistic regression with incomplete covariate and auxiliary information. J Multivar Anal 2010 Nov;101(10):2389-2397.
- 18. Zhang Q, Zhang M, Chen Y, Zhu S, Zhou W, Zhang L, et al. Smoking status and cognitive function in a national sample of older adults. Front Psychiatry 2022 Jul;13:926708.
- 19. Stramecki F, Kotowicz KD, Piotrowski P, Frydecka D, Rymaszewska J, Beszłej JA, et al. Assessment of the association between cigarette smoking and cognitive performance in patients with schizophrenia-spectrum disorders: a case-control study. Front Psychiatry 2018 Dec;9:642.
- 20. Riaz T, Murtaza G, Arif A, Mahmood S, Sultana R, Al-Hussain F, et al. Nicotine smoking is associated with impaired cognitive performance in Pakistani young people. PeerJ 2021 Jun;9:e11470.
- 21. Chamberlain SR, Odlaug BL, Schreiber LR, Grant JE. Association between tobacco smoking and cognitive functioning in young adults. Am J Addict 2012 Nov;21(Suppl 1):S14-S19.
- 22. Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006 Mar;184(3-4):523-539.
- 23. Campos MW, Serebrisky D, Castaldelli-Maia JM. Smoking and cognition. Curr Drug Abuse Rev 2016;9(2):76-79.
- 24. Bhat TA, Kalathil SG, Bogner PN, Miller A, Lehmann PV, Thatcher TH, et al. Secondhand smoke induces inflammation and impairs immunity to respiratory infections. J Immunol 2018 Apr;200(8):2927-2940.
- 25. Nowak JK, Dybska E, Adams AT, Walkowiak J. Immune cell-specific smoking-related expression characteristics are revealed by re-analysis of transcriptomes from the CEDAR cohort. Cent Eur J Immunol 2022;47(3):246-259.
- 26. Muhammad F, Arpin W, Pratama YS, Lestari CH, Budianto P, Danuaji R, et al. A rare case of Fahr’s syndrome with dissociative amnesia. J. Keperawatan Padjadjaran 2022;10(3):224-227.
- 27. Leonard BE, Myint A. Changes in the immune system in depression and dementia: causal or coincidental effects? Dialogues Clin Neurosci 2006;8(2):163-174.
- 28. Wang K, Yang J, Zhang S, Wei D, Hao X, Tu S, et al. The neural mechanisms underlying the acute effect of cigarette smoking on chronic smokers. PLoS One 2014 Jul;9(7):e102828.
- 29. Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta-analysis. Ann Med 2012 Sep;44(6):588-597.
- 30. Jamal H, Abdullah AZ, Abdullah MT. Determinan sosial perilaku merokok pelajar di Indonesia: analisis data global youth tobacco survey tahun 2014. J Kesehat Vokasional 2020;5(3):141-150.
- 31. Sharp ES, Gatz M. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord 2011;25(4):289-304.
- 32. Singh P, Goncalves I, Tengryd C, Nitulescu M, Persson AF, To F, et al. Reduced oxidized LDL in T2D plaques is associated with a greater statin usage but not with future cardiovascular events. Cardiovasc Diabetol 2020 Dec;19(1):214.
- 33. Danuaji R, Suroto S, Purwanto B, Indarto D, Muhammad F, Mirawati DK, et al. Association between carotid intima media thickness and acute ischemic stroke at an Indonesian tertiary referral hospital. J Taibah Univ Med Sci 2022 Dec;18(4):771-777.
- 34. Kupelian V, Link CL, McKinlay JB. Association between smoking, passive smoking, and erectile dysfunction: results from the Boston area community health (BACH) survey. Eur Urol 2007 Aug;52(2):416-422.
- 35. Indonesia, Badan Pusat Statistik. Literacy rate of population aged 15 years and over by province (Percent), 2023. 2023 [cited 2024 August 7]. Available from: https://www.bps.go.id/en/statistics-table/2/MTQ1OCMy/literacy-rates-among-population-aged-15-years-and-above-by-province.html.