Polyangiitis overlap syndrome (POS) is a disease entity proposed by Leavitt and Fauci in 1986.1 It is characterized as a systemic vasculitis that does not fit into one of the well-defined vasculitic syndromes. Here, we present a case of POS with a combination of polyarteritis nodosa (PAN) and idiopathic leukocytoclastic vasculitis (LCV), along with cardiopulmonary involvement and vocal cord palsy. The patient achieved successful remission of the vasculitis with the use of mycophenolate mofetil (MMF) and corticosteroids without any relapse.
Case Report
A 38-year-old Malay man with no known comorbidities presented with a three-month history of progressive dyspnea, intermittent fever, and a rash eruption on his bilateral lower limbs. Initially, he was diagnosed with community-acquired pneumonia and started on intravenous co-amoxiclav and azithromycin. However, his condition progressively worsened, with persistent pyrexia, tachypnoea, and tachycardia. Given this deterioration, further investigations were conducted to rule out deep-seated infections, tuberculosis, and pulmonary embolism.
Laboratory findings revealed a prominent leukocytosis at 26.2 × 103/µL (with neutrophils of 92.6% of the total count). The patient's C-reactive protein and N-terminal pro b-type natriuretic peptide were markedly elevated at 110.12 mg/L and 3203 pg/mL (< 450 pg/mL), respectively. Other laboratory investigations yielded unremarkable results, except for low serum albumin levels (29 g/L).
A computed tomography pulmonary angiogram ruled out pulmonary embolism but revealed collapse-consolidation of the right perihilar region with a moderate pleural effusion [Figure 1]. Electrocardiography showed sinus tachycardia with a poor R-wave progression. Transthoracic echocardiography revealed a grossly dilated left atrium and left ventricle with a left ventricular ejection fraction of 21% and the presence of a left ventricular thrombus. No valvular abnormalities were noted [Figure 2]. Computed tomography coronary angiography revealed a 50–75% stenosis in the left anterior descending artery (without aneurysm formation).
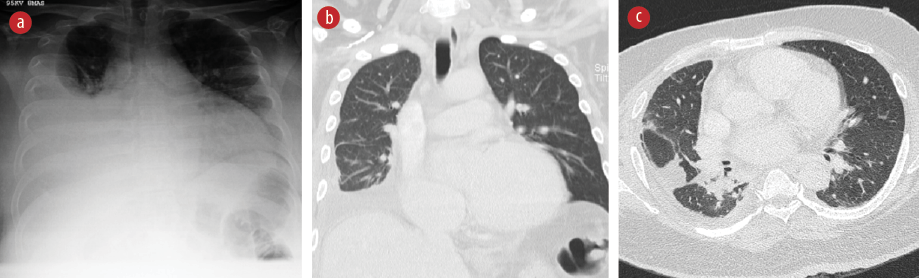 Figure 1: (a) Posteroanterior view chest radiograph showing a right pleural effusion with enlarged cardiac silhouette. (b) Coronal section CT scan of the thorax showing residual right pleural effusion with loculation in the right oblique and transverse fissures. (c) Transverse section of the CT thorax shows collapse consolidation in the right perihilar region, extending to the posterobasal and lateral basal segments.
Figure 1: (a) Posteroanterior view chest radiograph showing a right pleural effusion with enlarged cardiac silhouette. (b) Coronal section CT scan of the thorax showing residual right pleural effusion with loculation in the right oblique and transverse fissures. (c) Transverse section of the CT thorax shows collapse consolidation in the right perihilar region, extending to the posterobasal and lateral basal segments.
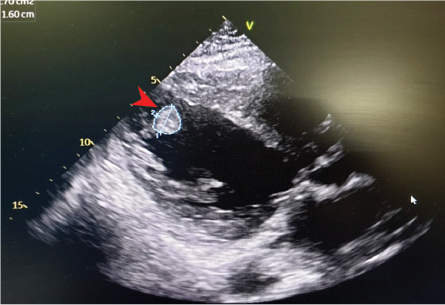 Figure 2: 2D echocardiography shows dilated
Figure 2: 2D echocardiography shows dilated
left atrial and left atrial (LV) with apical LV thrombus (arrowhead).
Despite repeated negative blood cultures and an extensive evaluation for infection, including the SARS CoV-2/COVID-19 RNA detection, anti-hepatitis C virus antibody, hepatitis B surface antigen, HIV I/II antigen/antibodies, and venereal disease research laboratory, no infectious etiology was identified. GeneXpert™ for Mycobacteria tuberculosis from blood was also negative. Pleural fluid analysis showed an exudative nature with reactive leukocytosis (1165/µL neutrophils and 365/µL lymphocytes). Given the negative infective screen and clinical deterioration, systemic vasculitis was strongly suspected.
Upon re-evaluation, the patient denied symptoms such as chest pain, weight loss, anorexia, night sweats, and other signs suggestive of an underlying connective tissue disease. He received his COVID-19 vaccination four months before his initial symptoms. He denied using over-the-counter or traditional medications.
On clinical examination, the patient exhibited dysphonic speech and had a temperature of 38.3oC. His blood pressure was 132/92 mmHg, pulse rate was 132 beats/min (regular), respiratory rate was 30 breaths/min, and oxygen saturation was 96% on room air. Generalized petechiae and palpable purpura with multiple necrotic ulcers were observed on both legs [Figure 3]. No peripheral lymphadenopathy or uveitis was detected. Except for reduced air entry over the right lung base, the remainder of the physical examination was unremarkable. Nasoendoscopy revealed left vocal cord palsy without any lesions throughout the nasal passages, nasopharynx, and hypopharynx, suggesting left recurrent laryngeal neuropathy.
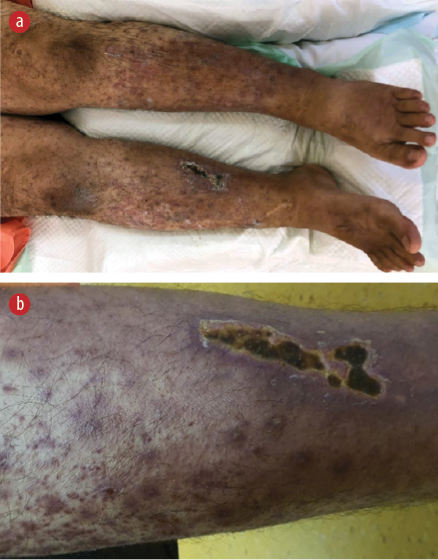 Figure 3: (a) Extensive purpuric rashes with ulcers on both lower limbs. (b) Close-up view.
Figure 3: (a) Extensive purpuric rashes with ulcers on both lower limbs. (b) Close-up view.
Autoimmune serology, including tests for ANA, anti-double stranded DNA, p- and c-antineutrophil cytoplasmic antibodies, C3 and C4, extractable nuclear antigens, myositis specific antibodies, and the paraneoplastic screen, did not reveal any significant findings. Unfortunately, the patient declined catheter angiography to assess for visceral vasculitis. Biopsy of the skin eruptions showed prominent fibrinoid necrosis in the superficial dermal vessels, with neutrophilic infiltration and LCV. The medium-sized vessels in the subcutaneous fat appeared normal, and immunofluorescence studies yielded negative results [Figure 4]. These findings were consistent with cutaneous LCV.
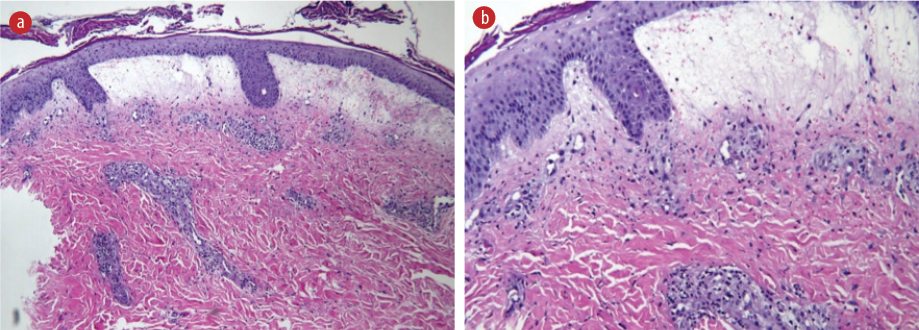 Figure 4: (a) Hematoxylin and eosin-stained section from skin biopsy shows focal hyperkeratosis with subepidermal blisters filled with blood and infiltrated by neutrophils. The blood vessels exhibit necrosis with foci of neutrophils and nuclear debris, indicating prominent fibrinoid necrosis. Red blood cells extravasations and perivascular collections of mixed inflammatory cells are present. The medium-sized vessel at the subcutaneous fat shows no vasculitis. Immunoglobulin (Ig)G, IgA, IgM, and C3 were negative, magnification = 20 ×. (b) Magnified view (40 ×) of the skin biopsy.
Figure 4: (a) Hematoxylin and eosin-stained section from skin biopsy shows focal hyperkeratosis with subepidermal blisters filled with blood and infiltrated by neutrophils. The blood vessels exhibit necrosis with foci of neutrophils and nuclear debris, indicating prominent fibrinoid necrosis. Red blood cells extravasations and perivascular collections of mixed inflammatory cells are present. The medium-sized vessel at the subcutaneous fat shows no vasculitis. Immunoglobulin (Ig)G, IgA, IgM, and C3 were negative, magnification = 20 ×. (b) Magnified view (40 ×) of the skin biopsy.
The patient’s condition demonstrates overlapping features of PAN and cutaneous LCV based on the clinical features of mononeuropathy, elevated diastolic blood pressure, and occlusive coronary artery lesion, with cutaneous LCV. A diagnosis of POS was made.
Because of the patient's clinical deterioration, treatment using high-dose corticosteroids and cyclophosphamide (CYC) infusion was planned. However, he declined CYC induction. He was initiated on intravenous methylprednisolone 500 mg daily for three days, in combination with MMF 500 mg twice daily. Additionally, rivaroxaban 15 mg twice daily was given for his intracardiac thrombus.
He had marked symptomatic improvement, with resolution of pyrexia, normalization of physiological parameters, and clearance of cutaneous lesions within three to four days. Subsequently, he was discharged on oral prednisolone 40 mg daily and MMF 500 mg daily.
At two months post-discharge, he remained well with the resolution of his dysphonia, pleural effusion, and cutaneous eruptions. His repeat hematological and biochemistry profile normalized. Given the good response to immunosuppression, he was put on a steroid taper and maintained on MMF 500 mg daily. Unfortunately, he defaulted on follow-up and treatment, leading to the development of decompensated heart failure, and eventually succumbed to the illness.
Discussion
The clinical presentation of the patient fulfills the diagnostic criteria for PAN.2 Although rare, bronchial artery vasculitis remains a recognized feature in PAN. He most likely had bronchial arteritis causing segmental pulmonary infarcts resulting in a collapse-consolidation and pleural effusion. The delayed diagnosis of coronary artery occlusion resulted in permanent cardiac insufficiency. The histopathological findings of small vessel vasculitis were consistent with the diagnosis of POS, which encompasses overlapping features of multiple vasculitic syndromes.
While there have been two reported cases of POS with a combination of PAN and idiopathic LCV, Our patient’s presentation is unique due to the involvement of the cardiopulmonary system and laryngeal neuropathy.1,3 Previous cases have utilized CYC in combination with high-dose corticosteroids for remission induction. In contrast, our patient was successfully treated with MMF in conjunction with corticosteroids.
Currently, there are no existing treatment guidelines for POS, and the management approaches described in the literature. However, the combination of CYC with high-dose corticosteroids is the most commonly used.1,4
The presumed T cell-mediated nature of the PAN, supported by the absence of associated autoantibodies, provides a rationale for using CYC as a therapeutic agent. CYC functions as a DNA-alkylating agent, impairing T-cell division and inducing apoptosis.5 MMF acts to deplete lymphocytic guanine nucleotides, producing effects similar to CYC.6
MMF has demonstrated effectiveness in remission induction for PAN, particularly in the pediatric population. Due to its relatively lower toxicity profile compared to CYC, MMF may be considered an acceptable alternative for induction therapy in POS.
Conclusion
POS is a systemic vasculitis that does not meet the diagnostic criteria for well-defined vasculitic syndromes. Timely immunosuppressive treatment is crucial in managing this condition to prevent vasculitic complications and irreversible organ dysfunction.
Disclosure
The authors declared no conflicts of interest. Informed consent was obtained from the patient’s wife.
references
- 1. Leavitt RY, Fauci AS. Polyangiitis overlap syndrome. Classification and prospective clinical experience. Am J Med 1986 Jul;81(1):79-85.
- 2. Lightfoot RW Jr, Michel BA, Bloch DA, Hunder GG, Zvaifler NJ, McShane DJ, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum 1990 Aug;33(8):1088-1093.
- 3. Pischel KD, Zvaifler NJ. Simultaneous occurrence of polyarteritis nodosa and leukocytoclastic vasculitis. J Rheumatol 1984 Aug;11(4):542-544.
- 4. Shoda H, Kanda H, Tanaka R, Komagata Y, Misaki Y, Yamamoto K. Wegener’s granulomatosis with eosinophilia. Intern Med 2005 Jul;44(7):750-753.
- 5. Ahlmann M, Hempel G. The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother Pharmacol 2016 Oct;78(4):661-671.
- 6. Ritter ML, Pirofski L. Mycophenolate mofetil: effects on cellular immune subsets, infectious complications, and antimicrobial activity. Transpl Infect Dis 2009 Aug;11(4):290-297.