|
Abstract
Objective: Diabetes mellitus is the most common chronic endocrine disease worldwide. Intensive glycemic control plays an important role in decreasing morbidity and mortality rate of the disease. Preclinical studies have shown that biotin has an essential role in regulating blood glucose and serum lipid metabolism. This study aims to evaluate the effect of biotin on glycemic control and plasma lipids concentrations in type 1diabetic patients.
Methods: This randomized double-blind placebo-controlled clinical trial study was conducted 70 type 1 diabetic patients with an age range 5-25 years old with poorly controlled (glycosylated hemoglobin ≥8%). Subjects were randomly allocated into two groups. In the intervention group biotin (40 microgram/kg) was administered plus daily insulin, while the control group received placebo plus daily insulin regimen for three months. Laboratory tests including glycosylated hemoglobin (HbA1c), fasting blood sugar and plasma lipids were measured at the base and after 3 months.
Results: In this study, seventy patients were evaluated, 35 were allocated to each group. There were no statistically significant differences between age, gender, duration of diabetes, BMI and BP between the two groups (p>0.05). HbA1c in the intervention (biotin) group was 9.84±1.80 at base and after 3 months treatment, it declined to 8.88±1.73 (p<0.001). In the control group HbA1c at base was 9.39±1.58, after 3 months it increased to10.11± 1.68. There were statistically significant differences in the mean of HbA1c in both the biotin and the control groups (p<0.001). FBS in the biotin group at base was 275±65.76 mg/dl and after 3 months it had reduced to 226± 41.31 (p<0.001). There were statistically significant differences in the mean of total cholesterol, low density lipoprotein cholesterol and triglyceride between the two groups at the end of 3 months (p<0.05).
Conclusion: Results of this study showed that biotin administration as an adjuvant in addition to insulin regimen can improve glycemic management and decrease plasma lipids concentrations in poorly controlled type 1 diabetic patients.
Keywords: Type one diabetes mellitus; Biotin, Serum lipid; HbA1c.
Introduction
Diabetes mellitus (DM) is a common metabolic chronic disorder worldwide. The incidence of DM increases by 3-4% annually; type one diabetes mellitus is accompanied by long term complications, including nephropathy, retinopathy, neuropathy and cardiovascular disease.1 Good glycemic control is the most important approach in decreasing complications of diabetes. However, good glycemic control is not achieved by most patients despite daily insulin consumption, partucularly during childhood diabetes.
Biotin (vitamin B7) which was introduced as a gene regulator, can increase insulin secretion by affecting beta cells of pancreatic islets, as well as enhance glycolysis pathway, and improves insulin function in muscles by provoking guanylate cyclase activity.2-6 In-vitro model, studies have shown that biotin has a synergistic effect with insulin and insulin, resistance of receptors is reduced by biotin.7-9
Glucokinase expression at both transcriptional and translational levels is regulated by biotin in the liver and pancreas.6,7 Glucokinase is a key enzyme in glycolysis pathway that is induced by biotin; as a result, blood glucose, ketone bodies, triglycerides, and free fatty acids are regulated.8 Several studies have confirmed that biotin can improve glycemic control in experimental diabetic animals.5,6,9,10 The beneficial effect of biotin in hyperglycemia was observed in both types 1 and 2 diabetes.11,12 Also, studies have shown a relation between biotin and lipid metabolism,13-16 but human studies, especially on type 1 diabetes are limited. In the present study, the effect of oral biotin administration plus insulin on glycemic management and serum lipids concentration was assessed in type 1 diabetics for a 3 months period.
Methods
This randomized double-blind placebo-controlled clinical trial study was conducted at a general university hospital in Kermanshah, located in the western Iran, during 2008-2009. Poorly controlled1 type 1 diabetic patients (HbA1c ≥8%) age between 5 and 25 years with a history of diabetes for at least one year were studied and were randomly allocated into groups; the intervention group and thecontrol group.
Exclusion criteria included any history of renal and cardiac disease, seizure, corticosteroids consumption, broad-spectrum antibiotics and anticonvulsives. Patient with a history of hypoglycemic attack or ketoacidosis 3 months prior to the study were also excluded, and pregnancy. Prior to enrollment, all subjects were informed signed consent forms.
A structural questionnaire information pertaining to demographic data such as including age, sex, weight, height and blood pressure (BP) was completed by trained interviewers.
Subjects were randomized in a 1:1 ratio of active treatment to placebo. The randomization schedule was developed using a standardized computer software, the concealment codes were given to subjects by blinded personnel, and based on the codes, medication was prescribed. The randomization and concealment codes were archived by personnel who were unaffiliated with the study.
In the intervention group, patients were prescribed 40 microgram/kg biotin tablets (not exceeding 2 mg daily) once a day for three months in addition to current insulin regimen. The control group received placebo (tablets were similar to biotin in size, color, taste and manufactured by Iran pakhsh) plus current insulin regimen. Subjects were on insulin injection therapy twice a day.
The subjects were visited monthly, they themselves measured capillary blood glucose daily and in the event of side effects were contacted by telephone and also any change in consumption of biotin, placebo and antibiotics was reported by phone.
Subjects were emphasized not to insert any significant changes in their diet, daily insulin consumption and physical activity unless based on their physicians prescription. None of the subjects were on lipid-lowering medication.
Patients with critical status such as hypoglycemia and ketoacidosis, or irregular administration of biotin for more than 5 days per month, were excluded from the study.
Laboratory tests including primary outcome (glycosylated hemoglobin [HbA1c by Nycocard kits], fasting blood sugar [FBS] and secondary outcome:total cholesterol [Chol], high density lipoprotein cholesterol [HDL], low density lipoprotein cholesterol [LDL] and triglyceride [TG]) were measured (by Pars Azmoon kits) at the baseline just before randomization and after 3 months.
The data were analyzed by SPSS 16 software. Student t-test and paired t-test were used to compare the means of groups as appropriate. Chi-square test was used to compare gender. Data are presented as mean±standard deviation, mean difference±standard error difference, 95% confidence interval of the difference, or when indicated, as absolute number. A p value <0.05 was considered significant.
This study was approved by the Ethics committee of Kermanshah University of Medical Sciences and registered in IRCT, registration number: 201110027691N1.
Results
Eighty four type 1 diabetic patients were studied in this survey, in which 14 were excluded, because of irregular administration of biotin for more than 5 days per month, and finally 70 patients were evaluated. There was no report of any side effects between the two groups. Thirty-five patients were allocated to each group. Demographic data including age, gender, duration of diabetes, body mass index (BMI) and BP are illustrated in (Table 1).
The RCT flow diagram shows the progress of subjects through the study. (Fig.1)
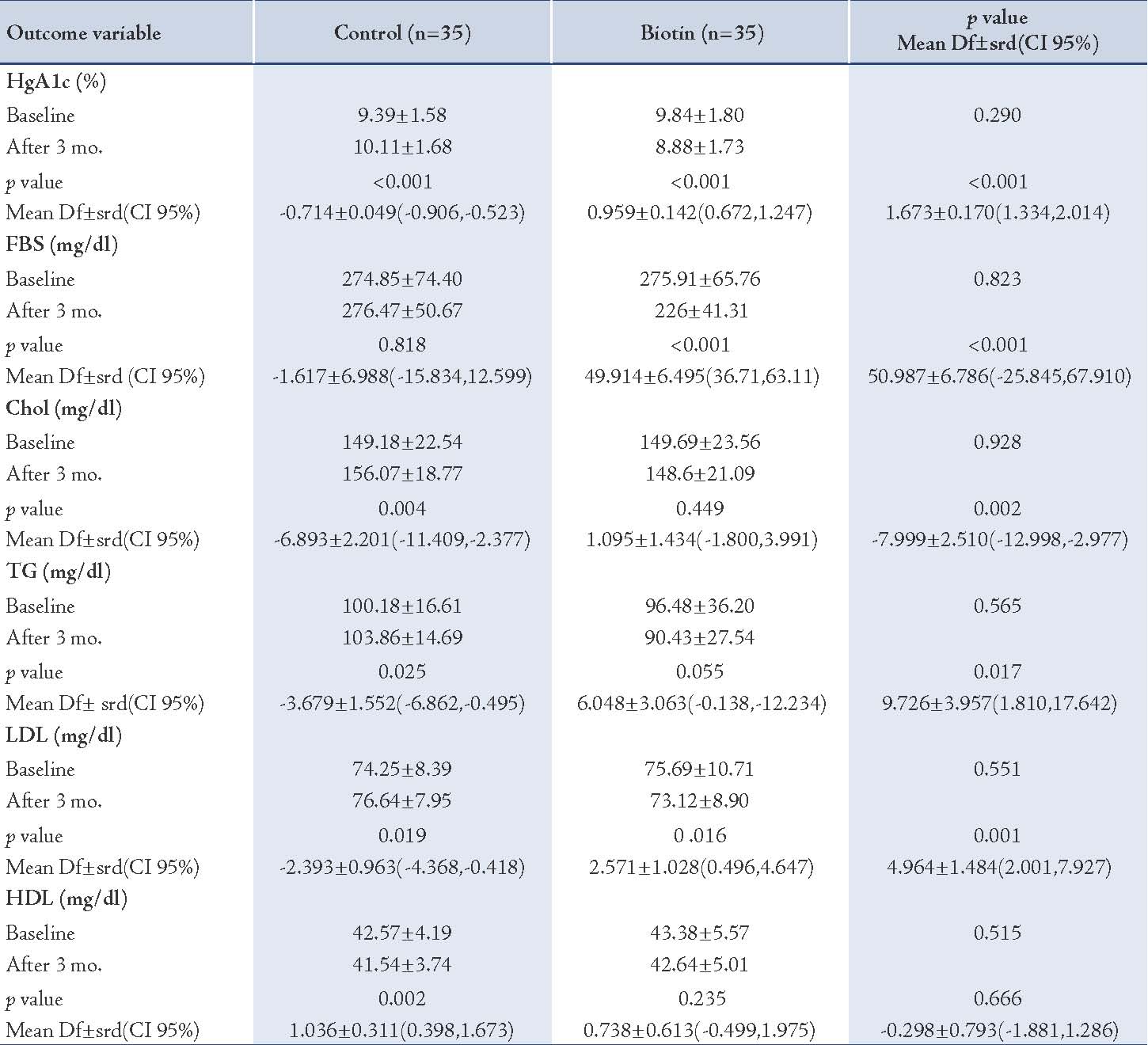
At the base, HbA1c in the intervention (biotin) group was 9.84±1.80 (mean±SD) and after 3 months it had declined to 8.88±1.73 (p<0.001). In the control group, HbA1c at the base was 9.39±1.58 and after 3 months it had increased to 10.11± 1.68. There was a statistically significant difference in the mean HbA1c in both biotin and the control groups (p<0.001). FBS in the biotin group was reduced considerably (p<0.001).
The mean plasma lipids concentration decreased in the biotin group, but only the reduction in concentration of LDL was significant (p=0.016). There were statistically significant differences in the mean value of total cholesterol, low density lipoprotein cholesterol and triglycerides between the two groups (p<0.05). (Table 2)
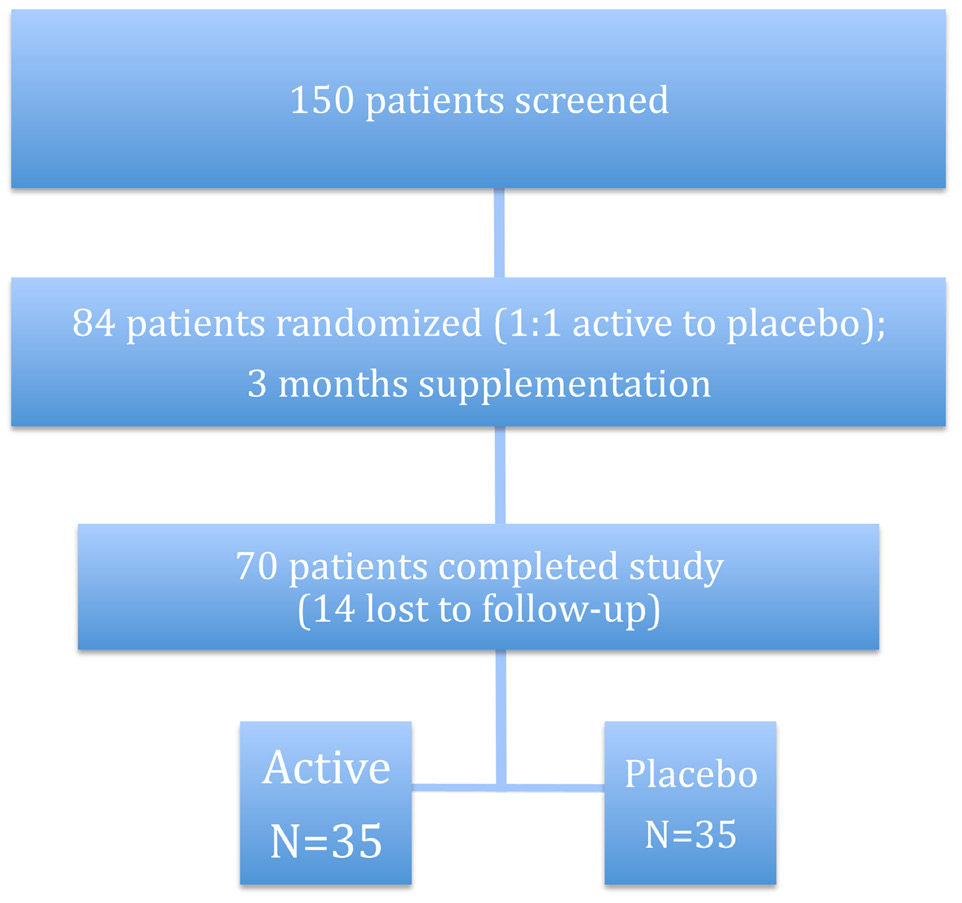
Figure 1: RCT study subject flow diagram.
Table 1: Demographic data.
Table 2: Laboratory Findings in Biotin and Control Groups.
Discussion
Results of this study showed that biotin administration as an adjuvant to current insulin therapy has a beneficial effect on glycemic control and reduces serum lipids concentrations in poorly controlled type 1 diabetic patients without any side effects.
The study findings supported previous studies, which have shown that biotin exhibits important effects on gene expression and improves glucose and lipid metabolism.3,4,10,17-20
Biotin is known to play an essential role in metabolic pathways such as gluconeogenesis and fatty acid synthesis by acting as a prosthetic group for carboxylases.2 Besides its role as a carboxylase prosthetic group, biotin exhibits principal effects on glucokinase expression in the liver and pancreas, glucokinase is a key enzyme in the glycolysis pathway which increases glucose catabolism and regulates blood glucose metabolism and plasma lipids concentrations.8,14 Also, one study on diabetic rats model showed biotin that repelled gluconeogenic genes and their transcription through a pathway independent of insulin signaling.21 Moreover, supraphysiological concentrations of biotin can activate guanylate cyclase, which promotes insulin function in muscles.9
Although the mechanism of hyperglycemia is different in type 1 and 2 diabetes, biotin is effective in both conditions.6-8,10,20,21 Coggeshall JC reported that daily administration of 16 mg biotin for 1 week decreased fasting blood sugar (FBS) up to 50% in type 1 diabetic patients.11Another study showed that administration of 9 mg biotin daily for 30 days reduced FBS up to 45% in type 2 diabetic patients.12 In hemodialysis patients, pharmacologic doses of biotin enhances their oral-glucose-tolerance tests.21 The current study results are in accordance with these findings suggesting biotin can decrease blood glucose; however, these studies did not measure HbA1c since the duration of intervention was shorter than 3 months. Also in recent studies, different doses of biotin were taken because the optimum dose of biotin in diabetes has not been determined precisely.
The present study shows that biotin significantly lowers plasma lipids compared with placebo. Our results are in accordance with other studies,15,16,22 which have demonstrated that biotin administration in diabetic patients decreases serum lipid concentrations. In contrast, a study on subjects with type 2 diabetes and non-diabetic subjects showed that biotin did not have any beneficial effect on glucose, insulin, triglyceride and cholesterol in either the diabetic or non-diabetic subjects.23 It seems that genetic and diet differences among subjects may account for these different responses to biotin.
Although the mechanism of action of biotin on lipid metabolism has not been definitively established, data from in-vivo study suggest that pharmacological doses of biotin decreases expression of messenger RNA of the lipogenic transcription factor in the liver.24
Several studies have revealed that a combination of biotin and chromium picolinate has a beneficial effect on glycemic control and lipid metabolism in type 2 diabetic patients.25,26 However, in these studies the combination of biotin and chromium picolinate was administered in non-dependent insulin diabetic patients. In a recent study, Albarracin CA evaluated the safety and tolerability of biotin and chromium picolinate and did not report any side effects.26
Conclusion
Biotin administered as an adjuvant in addition to insulin therapy can improve glycemic control, as well as serum lipids concentrations in type 1 diabetic patients without any side effects. However, further studies are needed with the larger samples to determine the effects of biotin as an adjuvant, in addition to insulin therapy on glycemic management and lipid metabolism in type 1 diabetic patients.
Acknowledgements
The authors would like to thank to Vice Chancellor of Research and Technology Center of Kermanshah University of Medical Sciences for funding.
References
1. Alemzadeh R, Wyatt DT. Diabetes Mellitus in children. In Kliegman R M et al.Nelson Text Book of Pediatrics.18th Edition. Philadelphia, 2007. p. 590.1-590.19.
2. Rodríguez Meléndez R. [Importance of biotin metabolism]. Rev Invest Clin 2000 Mar-Apr;52(2):194-199.
3. Fernandez-Mejia C. Pharmacological effects of biotin. J Nutr Biochem 2005 Jul;16(7):424-427.
4. Furukawa Y. [Enhancement of glucose-induced insulin secretion and modification of glucose metabolism by biotin]. Nihon Rinsho 1999 Oct;57(10):2261-2269.
5. Yoshikawa H, Tajiri Y, Sako Y, Hashimoto T, Umeda F, Nawata H. Effects of biotin on glucotoxicity or lipotoxicity in rat pancreatic islets. Metabolism 2002 Feb;51(2):163-168.
6. Romero-Navarro G, Cabrera-Valladares G, German MS, Matschinsky FM, Velazquez A, Wang J, et al. Biotin regulation of pancreatic glucokinase and insulin in primary cultured rat islets and in biotin-deficient rats. Endocrinology 1999 Oct;140(10):4595-4600.
7. McCarty MF. High-dose biotin, an inducer of glucokinase expression, may synergize with chromium picolinate to enable a definitive nutritional therapy for type II diabetes. Med Hypotheses 1999 May;52(5):401-406.
8. Ferre T, Pujol A, Riu E, Bosch F, Valera A. Correction of diabetic alterations by glucokinase. Proc Natl Acad Sci U S A 1996 Jul;93(14):7225-7230.
9. McCarty MF. cGMP may have trophic effects on beta cell function comparable to those of cAMP, implying a role for high-dose biotin in prevention/treatment of diabetes. Med Hypotheses 2006;66(2):323-328.
10. Zhang H, Osada K, Sone H, Furukawa Y. Biotin administration improves the impaired glucose tolerance of streptozotocin-induced diabetic Wistar rats. J Nutr Sci Vitaminol (Tokyo) 1997 Jun;43(3):271-280.
11. Coggeshall JC, Hegger JP, Robson MC, Baker H. Biotin status and plasma glucose in diabetics. Ann N Y Acad Sci 1985;447:389-393 .
12. Maebashi M, Mankino Y, Furukawa Y. Therapeutic evaluation of the effect of biotin on hyperglycemia in patient with NIDDM. Clin Biochem Nutr 1993;14:211-218 .
13. Marshall MW, Smith BP, Lehmann RP. Dietary response of two genetically different lines of inbred rats: lipids in serum and liver. Proc Soc Exp Biol Med 1969 Sep;131(4):1271-1277.
14. Scott D. Clinical biotin deficiency (egg white injury); report of a case with some remarks on serum cholesterol. Acta Med Scand 1958 Sep;162(1):69-70.
15. Marshall MW, Kliman PG, Washington VA, Mackin JF, Weinland BT. Effects of biotin on lipids and other constituents of plasma of healthy men and women. Artery 1980;7(4):330-351.
16. Dokusova OK, Krivoruchenko IV. [The effect of biotin on the level of cholesterol in the blood of patients with atherosclerosis and essential hyperlipidemia]. Kardiologiia 1972 Dec;12(12):113.
17. Fiume MZ; Cosmetic Ingredient Review Expert Panel. Final report on the safety assessment of biotin. Int J Toxicol 2001;20(Suppl 4):1-12.
18. Larrieta E, Velasco F, Vital P, López-Aceves T, Lazo-de-la-Vega-Monroy ML, Rojas A, et al. Pharmacological concentrations of biotin reduce serum triglycerides and the expression of lipogenic genes. Eur J Pharmacol 2010 Oct;644(1-3):263-268.
19. Vilches-Flores A, Tovar AR, Marin-Hernandez A, Rojas-Ochoa A, Fernandez-Mejia C. Biotin increases glucokinase expression via soluble guanylate cyclase/protein kinase G, adenosine triphosphate production and autocrine action of insulin in pancreatic rat islets. J Nutr Biochem 2010 Jul;21(7):606-612.
20. Sugita Y, Shirakawa H, Sugimoto R, Furukawa Y, Komai M. Effect of biotin treatment on hepatic gene expression in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem 2008 May;72(5):1290-1298.
21. Koutsikos D, Fourtounas C, Kapetanaki A, Agroyannis B, Tzanatos H, Rammos G, et al. Oral glucose tolerance test after high-dose i.v. biotin administration in normoglucemic hemodialysis patients. Ren Fail 1996 Jan;18(1):131-137.
22. Revilla-Monsalve C, Zendejas-Ruiz I, Islas-Andrade S, Báez-Saldaña A, Palomino-Garibay MA, Hernández-Quiróz PM, et al. Biotin supplementation reduces plasma triacylglycerol and VLDL in type 2 diabetic patients and in nondiabetic subjects with hypertriglyceridemia. Biomed Pharmacother 2006 May;60(4):182-185.
23. Báez-Saldaña A, Zendejas-Ruiz I, Revilla-Monsalve C, Islas-Andrade S, Cárdenas A, Rojas-Ochoa A, et al. Effects of biotin on pyruvate carboxylase, acetyl-CoA carboxylase, propionyl-CoA carboxylase, and markers for glucose and lipid homeostasis in type 2 diabetic patients and nondiabetic subjects. Am J Clin Nutr 2004 Feb;79(2):238-243.
24. Fernandez-Mejia C, Lazo-de-la-Vega-Monroy ML. Biological effects of pharmacological concentrations of biotin. JEBCAM 2011;16:40-48.
25. Singer GM, Geohas J. The effect of chromium picolinate and biotin supplementation on glycemic control in poorly controlled patients with type 2 diabetes mellitus: a placebo-controlled, double-blinded, randomized trial. Diabetes Technol Ther 2006 Dec;8(6):636-643.
26. Albarracin CA, Fuqua BC, Evans JL, Goldfine ID. Chromium picolinate and biotin combination improves glucose metabolism in treated, uncontrolled overweight to obese patients with type 2 diabetes. Diabetes Metab Res Rev 2008 Jan-Feb;24(1):41-51.
|