Kidney transplantation is the preferred treatment for patients with end-stage kidney disease as it offers better odds of long-term survival and a higher quality of life compared to dialysis. The risks include post-transplantation complications, leading to allograft dysfunction and eventual graft failure.1 Early post-transplant complications can range from slow graft function (SGF) and delayed graft function (DGF) to rejection of the transplanted kidney.2
DGF is defined by the Organ Procurement and Transplantation Network and the United Network for Organ Sharing as the need for dialysis within the first week of transplant and is associated with an extended hospital stay, a higher rejection risk, and poorer long-term survival of the graft.1,3,4 Recipients of extended donor criteria kidneys and kidneys donated after cardiac death are more likely to experience DGF.5,6 The incidence of DGF ranges 4–10% for transplants from living donors and 19–70% for transplants from deceased donors.7,8 SGF, an intermediate phenotype, is defined as a slower initial postoperative fall in serum creatinine without the requirement for dialysis.9,10 Despite advances in transplantation, DGF remains an obstacle towards good transplant outcomes, and currently, there is no effective treatment except to attempt to limit further allograft injury, for which early identification of the graft status is essential.
Clinically, DGF is a manifestation of acute kidney injury (AKI), the most common cause being post-transplantation acute tubular necrosis.11 To predict such a possibility, it is essential to identify an early biomarker of AKI with good sensitivity and specificity. Although serum creatinine is a conventional marker, it is nonspecific as many other factors may influence its reliability, and by the time an increase in serum creatinine is noticed, graft dysfunction would have already developed. Years of efforts have identified a few novel biomarkers with the potential to facilitate early detection of DGF. Among these, neutrophil gelatinase-associated lipocalin (NGAL), an acute-phase protein, has shown promise as an indicator of proximal tubular injury.12 In response to nephrotoxic injury NGAL expression increases in renal tubular cells, blood, and urine. Thus, NGAL levels can predict both the short- and long-term prognoses of transplant recipients and possibly facilitate early detection of graft dysfunction.13
Elevated production of NGAL by the tubular epithelium in allografts may occur due to potential stresses to the donated kidney at different stages: before its withdrawal from the donor, during ischemic storage, and during reperfusion.14 In previous studies, NGAL started to rise at two hours after injury, peaked at 8–12 hours, and returned to normal after 24–48 hours; however, the above timeline is based on limited data.15,16 Further, the predictive reliability of NGAL levels in both serum and urine needs to be established. It is still unclear whether urine NGAL or serum NGAL is better at predicting the onset of DGF. There is also scarce data on their predictive value in patients in developing countries. Therefore, the current study aimed to assess the accuracy of both serum and urine NGAL in predicting DGF in a group of kidney transplant recipients in South India.
Methods
This prospective observational cohort study was conducted from January 2018 to December 2022 at a tertiary care hospital in Southern India. The study received approval from the Kasturba Medical College and Kasturba Hospital Institutional Ethics Committee (IEC-68/2017), and all participants provided informed consent. This study included kidney recipients of both sexes who were > 18 years of age and had received kidneys from living-related or deceased donors. Patients with any medical condition that could interfere with NGAL measurements, such as active infection and sepsis, were excluded. The study adhered to the reporting guidelines of Strengthening the Reporting of Observational Studies in Epidemiology.
The demographic data collected included age, sex, and body mass index. The cause for renal failure, type of renal transplantation, and induction therapy used were included. The post-transplantation serum creatinine was measured daily and serum creatinine reduction on day 2 (CRR2) and on day 7 (CRR7) were calculated.5 The serum NGAL and urine NGAL were quantitatively measured using a standardized one-step test kit. For urine NGAL analysis, 8 mL urine was centrifuged at 3500 rpm for 5 min to avoid particulate matter and cell debris, and for serum NGAL, 2.4 mL blood was used to separate the serum. We used FIA8000 Quantitative Immunoassay Analyzer (Getein Biotech, Inc.) which uses colloidal gold immunochromatography to determine serum and urine NGAL levels. The graft function categories immediate graft function, SGF, and DGF were defined to categorize the recipients [Table 1].
Table 1: Definitions of post-transplant graft function categories.
|
Immediate graft function
|
CRR2 > 30% and CRR7 > 70%
|
|
Slow graft function
|
CRR2 ≤ 30%, or CRR7 ≤ 70%, or ≤ 10% per day fall in creatinine in the first week post-transplant*
|
CRR2: Serum creatinine reduction on day 2; CRR7: Serum creatinine reduction on day 7.
The normality was assessed by the Shapiro test. For continuous data, mean±SD were used. Non-continuous variables were summarized using the median with the IQR. To compare the percentages of categorical variables, either the chi-square test or the Fisher exact test was utilized. We used a t-test to compare the means of continuous variables, and a Mann-Whitney U test to compare the medians. Receiver operating characteristic (ROC) curve analysis was employed to determine the optimal sensitivity and specificity of urine and serum NGAL in predicting DGF. For statistical analysis, we used SPSS (IBM Corp. Released 2023. IBM SPSS Statistics for Windows, Version 29.0.2.0 Armonk, NY: IBM Corp). Statistical significance was set at p-value < 0.05.
Results
Based on the inclusion criteria, 40 renal transplant recipients participated in the study. The majority (24; 60.0%) were categorized as IGF, four (10.0%) as DGF, and 12 (30.0%) as SGF [Figure 1]. Due to the small sample size, we combined the SGF and DGF groups for the analysis. The overall mean age of the participants was 40.0±11.0 years. Group-wise, the mean age was 24.0±11.3 years for IGF, and 34.0±11.2 years for SGF±DGF. The overall mean age was 49.0±10.6 years for donors and 40.0±11.0 years for recipients. The vast majority (36; 90.0%) of transplant recipients were male of whom 22/36 were classified as IGF and 14/36 as SGF+DGF. Chronic glomerulonephritis was the leading cause of renal failure in both groups. There were on average 3/6 human leukocyte antigen mismatches in this study across both groups [Table 2].
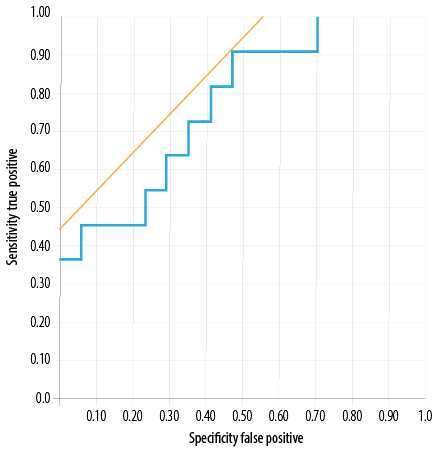 Figure 1: Receiver operating-characteristic curves for serum neutrophil gelatinase-associated lipocalin at two hours post-transplantation for predicting graft function in the slow and delayed graft
Figure 1: Receiver operating-characteristic curves for serum neutrophil gelatinase-associated lipocalin at two hours post-transplantation for predicting graft function in the slow and delayed graft
functions group.
Table 2: Baseline characteristics of the transplant recipients.
|
Recipient age*, years
|
40.0 ± 11.0
|
24.0 ± 11.3
|
34.0 ± 11.2
|
0.600
|
|
Male recipient, n (%)
|
36 (90.0)
|
22 (91.7)
|
14 (87.5)
|
0.700
|
|
Donor age*, years
|
49.0 ± 10.6
|
42.0 ± 8.3
|
45.0 ± 10.7
|
0.800
|
|
Chronic glomerulonephritis, n (%)
|
28 (70.0)
|
18 (75.0)
|
10 (62.5)
|
0.300
|
|
HLA mismatches*
|
3.3 ± 1.3
|
3.3 ± 1.0
|
3.3 ± 1.2
|
0.600
|
|
Donor type, n (%)
|
|
Cadaveric
|
6 (15.0)
|
2 (8.3)
|
4 (25.0)
|
0.040†
|
|
Live donor
|
34 (85.0)
|
22 (91.6)
|
12 (75.0)
|
0.010†
|
|
Induction agents, n (%)
|
|
ATG
|
7 (17.5)
|
2 (8.3)
|
5 (31.3)
|
0.900
|
|
ATG-F
|
9 (22.5)
|
4 (16.7)
|
5 (31.3)
|
0.200
|
|
Basiliximab
|
24 (60.0)
|
18 (75.0)
|
6 (37.5)
|
0.400
|
|
Serum creatinine*, mg/dL
|
|
Day 1
|
3.9 ± 1.6
|
2.8 ± 1.2
|
5.2 ± 1.8
|
0.010†
|
|
Day 2
|
2.3 ± 0.8
|
1.6 ± 0.6
|
5.0 ± 1.9
|
0.010†
|
IGF: immediate graft function; DGF: delayed graft function; SGF: slow graft function; HLA: human leukocyte antigen; ATG: antithymocyte globulin;
ATG-F: antithymocyte globulin-fresenius. *Mean ± SD; †: Significance.
Most 34/40 (85.0%) kidney recipients underwent live-related renal transplantation. Cadaveric transplants were significantly more in the SGF+DGF group (p = 0.040) while live-related kidney transplants were significantly more in the IGF group (p = 0.001). The induction agents used were basiliximab, antithymocyte globulin (ATG), and ATG-Fresenius (ATG-F). The majority received basiliximab (60.0%), and there was no difference in the use of induction agents between groups
(p > 0.050). The mean serum creatinine value was significantly higher in the SGF+DGF group compared to the SGF group (p = 0.010) [Table 2].
In our study, we analyzed the median concentrations of serum and urine NGAL at two hours post-transplantation. In the SGF+DGF group, both serum and urine NGAL were higher compared to the IGF group (807 vs. 620 ng/mL, p = 0.020; 590 vs. 476 ng/mL, p = 0.040) [Table 3].
Serum NGAL demonstrated a sensitivity of 90.0% and specificity of 53.0% at a cut-off value of 678 ng/mL. The area under the ROC curve was 0.77 [Figure 1]. While urine NGAL showed 70.0% sensitivity and 74.0% specificity at cut-off 489 ng/mL, the area under the ROC curve was 0.72 [Figure 2]. Thus, in our cohort, serum NGAL was more sensitive than urine NGAL in predicting SGF/DGF; however, as far as specificity was considered both were found to be non-specific.
Table 3: Serum and urine NGAL at two hours post-transplantation.
|
Serum NGAL*, ng/mL
|
620 (184.7–2586.6)
|
807 (317.0–1662.0)
|
0.020
|
NGAL: neutrophil gelatinase-associated lipocalin; IGF: immediate graft function; SGF: slow graft function; DGF: delayed graft function.
*Median ± IQR.
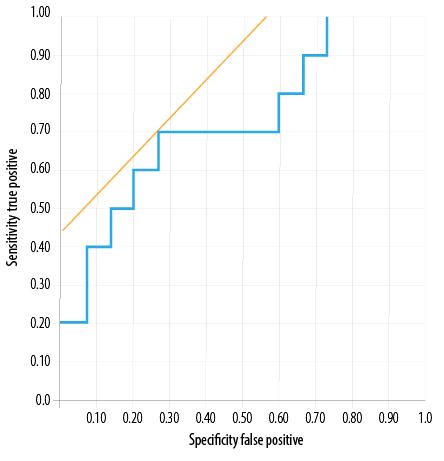 Figure 2: Receiver operating-characteristic curves for urine neutrophil gelatinase-associated lipocalin at two hours post-transplantation for predicting graft function in the slow and delayed graft functions group.
Figure 2: Receiver operating-characteristic curves for urine neutrophil gelatinase-associated lipocalin at two hours post-transplantation for predicting graft function in the slow and delayed graft functions group.
Discussion
NGAL is a small group of secretory glycoproteins comprising of 20 amino acids at the N-terminal end followed by a ‘lipocalin’ domain of 48–193 amino acids.12 Initially, NGAL was identified as a component of neutrophil granules; however, it is also secreted from several human tissues including hepatocytes, and gastrointestinal and pulmonary tract cells.17 According to reports, it participates in several pathways, including apoptosis, bacteriostasis, proliferation, and regeneration of renal tubule epithelial cells.14 As NGAL levels rise significantly in response to proximal tubular injury, both urine and serum NGAL have predictive value in various clinical conditions of AKI, including cardiac surgery,18 and contrast-induced AKI after cardiac cauterization.19 However, a literature review suggests that in the context of post-renal transplantation DGF, the predictive ability of NGAL may vary.20 To effectively detect DGF in kidney transplant recipients, clinicians must know when to detect NGAL which usually begins to rise two hours after the injury, peaks at 8–12 hours, and returns to baseline after 24–48 hours.21 In this study, we conducted a quantitative comparison of the predicting ability of serum and urine NGAL in recipients who underwent live-related as well as cadaveric renal transplantation and subsequently developed SGF or DGF.
Out of our 40 renal transplant recipients, 60.0% had IGF and 40.0% had SGF+DGF. These findings share similarities with the study by Hall et al,21 with 91 recipients (54.9% had deceased donors) of whom 37.3% developed DGF, 36.2% had SGF, and 26.3% had IGF. Pourmand et al,22 analyzed 29 post-transplant recipients among whom 27.6% of recipients developed DGF which is also similar to our finding.
In the current study, basiliximab was used as the induction agent in 60.0% of the overall study population. Similarly in a study on 170 recipients in Austria, 70% of the IGF group and 87% of the DGF group were given basiliximab as the induction agent.23 On the other hand, a study from the USA found that ATG was used in 56% of 92 deceased donor kidney transplant patients.21 Such differences in the choice of induction agents may be attributed to the lack of uniformity in the choice of induction agents among nephrologists as well as variations in recipient and donor status in different studies.
In the present study, the median concentration of both serum and urine NGAL at two hours were higher in the SGF+DGF group than in the IGF group. This aligns with a study by Hollmen et al,24 et al, where they quantified serum NGAL at intervals of < 6 hours, 7–12 hours, and > 12 hours and found higher mean serum NGAL level of 687 ng/mL in DGF patients at < 6 hours post-transplant and reported that DGF predictability was best in the group where the samples were drawn < 6 hours post-transplant, with a 436 ng/mL cut off. Furthermore, another study reported urine NGAL at 560 ng/mL on day one post-transplant.25 Pourmand et al,22 also found that serum NGAL predicted DGF during the early post-transplantation period (p = 0.005); further, serum NGAL was the only significant independent predictor of DGF based on the multivariate analysis (p = 0.039). In our study, the choice of measuring NGAL at two-hour post-transplantation turned out to be important, as it is the critical point in time for significant changes in NGAL production to occur, indicating the initial response to a renal injury. Quantifying NGAL at this point offers insights into the early stages of kidney injury, aiding timely detection and management.
In the current study, serum NGAL showed a sensitivity of 90.0% and specificity of 53.0% while urine NGAL showed 70.0% sensitivity and 74.0% specificity suggesting that, serum NGAL was more sensitive than urine NGAL in predicting SGF/DGF but with low specificity. This maybe because the predictability of urine NGAL might be affected by the frequent occurrence of oliguria and anuria in the postoperative period.15,16 However, a metanalysis by Li et al,20 comprising 14 observational studies (eight on urine NGAL and six on serum NGAL) with a total of 1036 kidney recipients, found that the composite AUC for urine NGAL (0.91; 95% CI: 0.89–0.94) had 88% sensitivity and 81% specificity, and the composite AUC for serum NGAL (0.95; 95% CI: 0.93–0.97) had 91% sensitivity and 86% specificity.20 Their results suggested that both urine and serum NGAL are acceptable biomarkers for early prediction of DGF.
Theoretically, urine NGAL would be expected to represent kidney injury better than serum NGAL. This is because most urine NGAL is produced by the distal nephrons rather than being filtered from the blood, while serum NGAL comes from the systematic pool.26 However, a meta-analysis study by Li et al,20 demonstrated that in practice serum NGAL has greater sensitivity and marginally higher specificity compared to urine NGAL. A Belgian study on 97 transplant patients also found that serum NGAL had higher AUC values than urine NGAL.27 A potential explanation is that a variety of variables including urine concentration and glomerular filtration rate could influence urine NGAL levels, decreasing urine NGAL predictive power. Thus, clinicians tend to prefer serum NGAL as the more practical predictor for kidney transplant recipients who are likely to experience severe oliguria or even anuria after surgery.
Table 4 lists previous studies with individual cut-off values on serum NGAL and urine NGAL for predicting renal graft dysfunction. The variations in sensitivity and specificity between the studies are likely to be due to multiple factors like differences in population, race, methods of estimation, and sampling timings.
Table 4: Overview of studies that analyzed urinary and serum NGAL levels to predict DGF.
|
Serum NGAL
|
|
|
|
|
|
|
|
|
Pezeshgi et al,28 Iran (2016)
|
37
|
6 hrs and 12 hrs
|
6 hrs: 309
12 hrs: 317
|
0.68
0.97
|
66
100
|
64
92
|
12 hrs NGAL plasma, NGAL showed higher predictability compared to serum creatinine
|
|
Cantaluppi et al,29 Italy (2015)
|
50
|
24 hrs
|
532
|
0.94
|
90.9
|
80.6
|
Significantly higher levels of NGAL in the DGF group than in the non-DGF group
|
|
Hollmen et al,24 Finland (2014)
|
176
|
< 6 hrs, 7–12 hrs, and
> 12 hrs
|
< 6 hrs: 436
7–12 hrs: 420
> 12 hrs: 420
|
1.00
0.86
0.92
|
100
94
91
|
100
73
|
DGF Predictability was best where sample was drawn < 6 hrs
|
|
Lee et al. Korea,30 (2012)
|
59
|
Day 1, 5, and 14. ROC was compared with IL-18 and SCr
|
Day 1: 233.3
|
0.86
|
78.6
|
77.8
|
The AUC of NGAL on day 1 was higher than IL-18 and creatinine. On days 5 and 14, the AUC values were not significant to predict DGF
|
|
Urinary NGAL
|
|
|
|
|
|
|
|
|
Nieto -Ríos et al,31 Columbia (2016)
|
79
|
48 hrs
|
120
|
0.80
|
75
|
71
|
NGAL at 48 hrs predicted DGF
|
|
Cui et al,32 China (2015)
|
129
|
At 4, 12, 24, 48, and 72 hrs. ROC was compared with IL-18 and SCr
|
4 hrs: 521.7
12 hrs: 559.2
24 hrs: 688.3
48 hrs: 295.2
72 hrs: 297.4
|
4 hrs: 0.78
12 hrs: 0.80
24 hrs: 0.83
48 hrs: 0.89
72 hrs: 0.86
|
4 hrs: 80.0
12 hrs: 80.0
24 hrs: 70.0
48 hrs: 80.0
72 hrs: 80.0
|
4 hrs: 68.7
12 hrs: 68.7
24 hrs: 93.7
48 hrs: 96.9
72 hrs: 100
|
The specificity was increased with time, but sensitivity does not show any change
AUC was higher to 0.984 with 100% sensitivity and 96.9% specificity in the combination panel of NGAL+ IL-18 and SCr
|
|
Kanter et al,33 Spain (2013)
|
38
|
At days 1, 3, 6, and 10.
Compared ROC between days 1 and 3
|
Day 1: 128
Day 3: 124
|
0.71
0.91
|
85.7
80
|
61.5
83
|
Day 3: uNGAL had better predictability than day1
|
|
Hollmen et al,25 Finland (2011)
|
176
|
Day 1
|
560
|
0.74
|
65
|
74
|
Day 1: uNGAL predicted early and prolonged DGF that led to the worst graft survival
|
|
Serum and urinary NGAL
|
|
|
|
|
|
|
|
NGAL: neutrophil gelatinase-associated lipocalin; DGF: delayed graft function; IL-18: Interleukin-18, SCr: serum creatinine; u NGAL: urinary NGAL; sNGAL: serum NGAL; ROC: receiver operating characteristic curve; AUC: area under the curve.
The main limitation of this study is that it was conducted at a single center with a relatively small sample size. More studies with larger sample sizes from different parts of India are required to generate nationally representative data. Second, we conducted only a single serum and urine NGAL measurement per patient and did not include serial monitoring of NGAL over time. Third, we did not compare the predictive performance of NGAL with other potential biomarkers, which could provide a more comprehensive understanding of their predictive abilities.
Conclusion
In our population, both serum and urine NGAL were significantly elevated in renal transplant recipients with SGF/DGF. The serum NGAL was more sensitive than urine NGAL for predicting SGF/DGF, but both had poor specificity. The optimum NGAL cut-off value for clinical application will need to be determined by more large-scale prospective cohort studies with serial monitoring over time to identify cut-off values of both serum and urine NGAL for predicting DGF.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Wu WK, Famure O, Li Y, Kim SJ. Delayed graft function and the risk of acute rejection in the modern era of kidney transplantation. Kidney Int 2015 Oct;88(4):851-858.
- 2. Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet 2004 Nov;364(9447):1814-1827.
- 3. Tapiawala SN, Tinckam KJ, Cardella CJ, Schiff J, Cattran DC, Cole EH, et al. Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol 2010 Jan;21(1):153-161.
- 4. Butala NM, Reese PP, Doshi MD, Parikh CR. Is delayed graft function causally associated with long-term outcomes after kidney transplantation? Instrumental variable analysis. Transplantation 2013 Apr;95(8):1008-1014.
- 5. Barreda Monteoliva P, Redondo-Pachón D, Miñambres García E, Rodrigo Calabia E. Kidney transplant outcome of expanded criteria donors after circulatory death. Nefrologia (Engl Ed) 2022;42(2):135-144.
- 6. Querard AH, Le Borgne F, Dion A, Giral M, Mourad G, Garrigue V, et al. Propensity score-based comparison of the graft failure risk between kidney transplant recipients of standard and expanded criteria donor grafts: toward increasing the pool of marginal donors. Am J Transplant 2018 May;18(5):1151-1157.
- 7. Querard AH, Foucher Y, Combescure C, Dantan E, Larmet D, Lorent M, et al. Comparison of survival outcomes between expanded criteria donor and standard criteria donor kidney transplant recipients: a systematic review and meta-analysis. Transpl Int 2016 Apr;29(4):403-415.
- 8. Melih KV, Boynuegri B, Mustafa C, Nilgun A. Incidence, risk factors, and outcomes of delayed graft function in deceased donor kidney transplantation. Transplant Proc 2019 May;51(4):1096-1100.
- 9. Boom H, Mallat MJ, de Fijter JW, Zwinderman AH, Paul LC. Delayed graft function influences renal function but not survival. Transplant Proc 2001;33(1-2):1291.
- 10. Moore J, Shabir S, Chand S, Bentall A, McClean A, Chan W, et al. Assessing and comparing rival definitions of delayed renal allograft function for predicting subsequent graft failure. Transplantation 2010 Nov;90(10):1113-1116.
- 11. Lechevallier E, Dussol B, Luccioni A, Thirion X, Vacher-Copomat H, Jaber K, et al. Posttransplantation acute tubular necrosis: risk factors and implications for graft survival. Am J Kidney Dis 1998 Dec;32(6):984-991.
- 12. Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta 2012 Aug;1826(1):129-169.
- 13. Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 2003 Oct;14(10):2534-2543.
- 14. Pajek J, Škoberne A, Šosterič K, Adlešič B, Leskošek B, Bučar Pajek M, et al. Non-inferiority of creatinine excretion rate to urinary L-FABP and NGAL as predictors of early renal allograft function. BMC Nephrol 2014 Jul;15:117.
- 15. Fonseca I, Oliveira JC, Almeida M, Cruz M, Malho A, Martins LS, et al. Neutrophil gelatinase-associated lipocalin in kidney transplantation is an early marker of graft dysfunction and is associated with one-year renal function. J Transplant 2013;2013:650123.
- 16. Chakraborty S, Kaur S, Tong Z, Batra SK, Guha S. Neutrophil gelatinase associated lipocalin: structure, function and role in human pathogenesis. InTech; 2011 Oct 5.
- 17. Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem 1993 May;268(14):10425-10432.
- 18. Ho J, Tangri N, Komenda P, Kaushal A, Sood M, Brar R, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis 2015 Dec;66(6):993-1005.
- 19. Zhou F, Luo Q, Wang L, Han L. Diagnostic value of neutrophil gelatinase-associated lipocalin for early diagnosis of cardiac surgery-associated acute kidney injury: a meta-analysis. Eur J Cardiothorac Surg 2016 Mar;49(3):746-755.
- 20. Li YM, Li Y, Yan L, Wang H, Wu XJ, Tang JT, et al. Comparison of urine and blood NGAL for early prediction of delayed graft function in adult kidney transplant recipients: a meta-analysis of observational studies. BMC Nephrol 2019 Aug;20(1):291.
- 21. Hall IE, Yarlagadda SG, Coca SG, Wang Z, Doshi M, Devarajan P, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol 2010 Jan;21(1):189-197.
- 22. Pourmand G, Emamjomeh B, Gooran S, Namdari F, Mehrsay A, Dehghani S, et al. The predictive value of serum NGAL for the diagnosis of delayed graft function in kidney transplantation. Translational Research in Urology 2021;3(4):182-190.
- 23. Maier HT, Ashraf MI, Denecke C, Weiss S, Augustin F, Messner F, et al. Prediction of delayed graft function and long-term graft survival by serum and urinary neutrophil gelatinase-associated lipocalin during the early postoperative phase after kidney transplantation. PLoS One 2018 Jan;13(1):e0189932.
- 24. Hollmen ME, Kyllönen LE, Merenmies J, Salmela KT. Serum neutrophil gelatinase-associated lipocalin and recovery of kidney graft function after transplantation. BMC Nephrol 2014 Jul;15:123.
- 25. Hollmen ME, Kyllönen LE, Inkinen KA, Lalla ML, Salmela KT. Urine neutrophil gelatinase-associated lipocalin is a marker of graft recovery after kidney transplantation. Kidney Int 2011 Jan;79(1):89-98.
- 26. Kuwabara T, Mori K, Mukoyama M, Kasahara M, Yokoi H, Saito Y, et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int 2009 Feb;75(3):285-294.
- 27. Buemi A, Musuamba F, Frederic S, Douhet A, De Meyer M, De Pauw L, et al. Is plasma and urine neutrophil gelatinase-associated lipocalin (NGAL) determination in donors and recipients predictive of renal function after kidney transplantation? Clin Biochem 2014 Oct;47(15):68-72.
- 28. Pezeshgi A, Abedi Azar S, Ghasemi H, Kamali K, Esmaeilzadeh A, Hajsalimi B, et al. Role of plasma neutrophil gelatinase-associated lipocalin as an emerging biomarker of acute renal failure following kidney transplantation and its correlation with plasma creatinine. J Renal Inj Prev 2016 Mar;5(2):98-103.
- 29. Cantaluppi V, Dellepiane S, Tamagnone M, Medica D, Figliolini F, Messina M, et al. Neutrophil gelatinase associated lipocalin is an early and accurate biomarker of graft function and tissue regeneration in kidney transplantation from extended criteria donors. PLoS One 2015 Jun;10(6):e0129279.
- 30. Lee EY, Kim MS, Park Y, Kim HS. Serum neutrophil gelatinase-associated lipocalin and interleukin-18 as predictive biomarkers for delayed graft function after kidney transplantation. J Clin Lab Anal 2012 Jul;26(4):295-301.
- 31. Nieto-Ríos JF, Serna-Higuita LM, Ocampo-Kohn C, Aristizábal-Alzate A, Vélez-Echeverry C, Vanegas-Ruiz JJ, et al. Neutrophil gelatinase-associated lipocalin as an early predictor of delayed graft function. Biomedica 2016 Jun;36(2):213-219.
- 32. Cui LY, Zhu X, Yang S, Zhou JS, Zhang HX, Liu L, et al. Prognostic value of levels of urine neutrophil gelatinase-associated lipocalin and interleukin-18 in patients with delayed graft function after kidney transplantation. Transplant Proc 2015 Dec;47(10):2846-2851.
- 33. Kanter J, Beltran S, Molina D, Vallecillo J, Sancho A, Gavela E, et al. Urinary neutrophil gelatinase-associated lipocalin after kidney transplantation: is it a good biomarker to assess delayed graft function? Transplant Proc 2013 May;45(4):1368-1370.