Heart failure (HF) can be considered a multi-organ pathology as its progression leads to dysfunction of internal organs due to hypoperfusion and systemic congestion. Systemic congestion is considered the main clinical feature of decompensation in HF.1,2 Eliminating this congestion is a main goal of therapy.3 Acute decompensated HF (ADHF) is the appearance or rapid aggravation of symptoms and signs of HF, which requires emergency hospitalization of the patient and intensive therapy in combination with objective signs of heart damage, and is associated with high mortality and repeated hospitalization, yet there is a lack of predictors for an unfavorable prognosis in ADHF patients.4,5 Of recent research interest is the prognostic significance of comprehensive evaluations using modern diagnostic methods including ultrasound to identify congestion in patients hospitalized with ADHF.6,7 Stratification of the risk of future cardiovascular events in HF patients may help identify high-risk patients who require enhanced therapy, thereby improving their long-term outcomes.8
Acute and chronic decompensated HF may be accompanied by hepatic dysfunction (cardio-hepatic syndrome),9 which is characterized by progressive liver fibrosis and declining synthetic function, associated with a worsening prognosis.10 A characteristic feature of cardio-hepatic syndrome that can be assessed by ultrasound is an increase in venous congestion, manifested by an expansion of the hepatic and portal veins and a change in the shape of blood flow. One promising and informative method is the comprehensive sequential ultrasound assessment of venous congestion comprising the venous excess ultrasound score (VExUS) protocol (which assesses the presence and severity of congestion), the study of port-hepatic Doppler waves, and measurement of the diameter of the inferior vena cava.11
The purpose of this study, therefore, was to evaluate the prognostic value (including survival and re-hospitalization) of ultrasound diagnostic methods including the number of B-lines using lung ultrasound, the presence of hepatic venous congestion according to the VExUS protocol, and liver density according to indirect elastometry, performed at the time of hospital discharge on patients with ADHF.
Methods
Decompensation of HF was diagnosed based on generally accepted criteria: the appearance or rapid aggravation of symptoms and signs of HF, requiring emergency hospitalization of the patient and intensive therapy in combination with objective signs of heart damage—systolic and/or diastolic dysfunction, left ventricular hypertrophy, left atrium enlargement according to echocardiography—and an increase in N-terminal pro-B-type natriuretic peptide (NT-proBNP).12
The study was conducted in the Clinical Hospital named after V.V. Vinogradov, Moscow, Russian Federation from February 2021 to Feruary 2023. This study excluded patients with acute coronary syndrome, lung diseases (exacerbation of chronic obstructive pulmonary disease, bronchial asthma), end-stage chronic kidney disease, malignant neoplasm, edematous syndrome of other etiology, primary liver pathology, acute hepatitis with increased transaminases (> 5 × the upper limits of normal), excessive alcohol consumption before hospitalization, severe cognitive deficit, or immobilization. Also excluded were patients on whom bioelectric impedance vector analysis could not be performed because of missing limbs, the presence of ulcers or pronounced trophic changes on the skin of the limbs, or the presence of metal implants and structures.
All patients signed an informed consent form to participate in the study. The study was approved by the Ethics Committee of the Peoples’ Friendship University of Russia (RUDN) Medical Institute, Moscow (Protocol No. 26 of 18.02.21).
All patients underwent a standard physical examination at admission and at discharge, as well as laboratory and instrumental studies, including NT-proBNP, lung ultrasound, indirect liver fibroelastometry, and Doppler ultrasound evaluation of hepatic venous congestion according to the VExUS protocol.
NT-proBNP in blood serum was determined by ELISA enzyme immunoassay using the NT-proBNP-ELISA-BEST test systems (Russia, Vector-Best CJSC). The accuracy of the test was 96.4% and was reliable.12
Upon discharge, ultrasound of both sides of the chest (GE Vivid E90, convex sensor) was performed in eight areas (II and IV m/r between the parasternal and midclavicular lines, and between the anterior and middle axillary lines). The number of B-lines was calculated, defined as vertical, hyperechoic reverberation artifacts from the pleural line to the bottom of the screen, moving synchronously with the movement of the lungs.
Indirect liver fibroelastometry was performed on the day of discharge using the FibroScan® 502 touch device (Echosens, France) according to a standard technique in the projection of the right lobe of the liver at the level of 8 or 9 intercostal space along the anterior or median axillary line.13 Studies with at least 10% and > 60% successful measurements were considered valid. The density (elasticity) of the liver in kilopascals (kPa) and the IQR in percent (%) were determined. The density quantitatively indicated the degree of fibrosis in this area of the liver parenchyma where the sensor was installed.
Ultrasound assessment of hepatic venous stagnation was carried out according to the VExUS protocol, on an expert-level device VIVIDTM E-90 (GE Healthcare) using abdominal and sector sensors, with an assessment of the diameter of the inferior vena cava, the shape of hepatic blood flows, and the portal in the mode of pulse-wave Dopplerography.14 The study of Doppler curves was carried out on exhalation with simultaneous electrocardiogram registration on the monitor of the ultrasound machine. The shape of the blood flow in the port-hepatic veins was consistently evaluated according to the VExUS protocol, with the inferior vena cava diameter at ≥ 2.0 cm.
When constructing receiver operating characteristic curves for predicting outcomes (total mortality + re-hospitalization), the threshold values for assessing congestion were set as follows: (a) the number of lines on lung ultrasound > 5 and (b) liver density > 6.2 kPa.
For hepatic vein Dopplerography, the systolic phase was normally of greater amplitude than the diastolic phase, while a decrease in systolic blood flow velocity was considered a minor deviation, and the presence of a reverse systolic phase was a pronounced deviation [Figure 1].
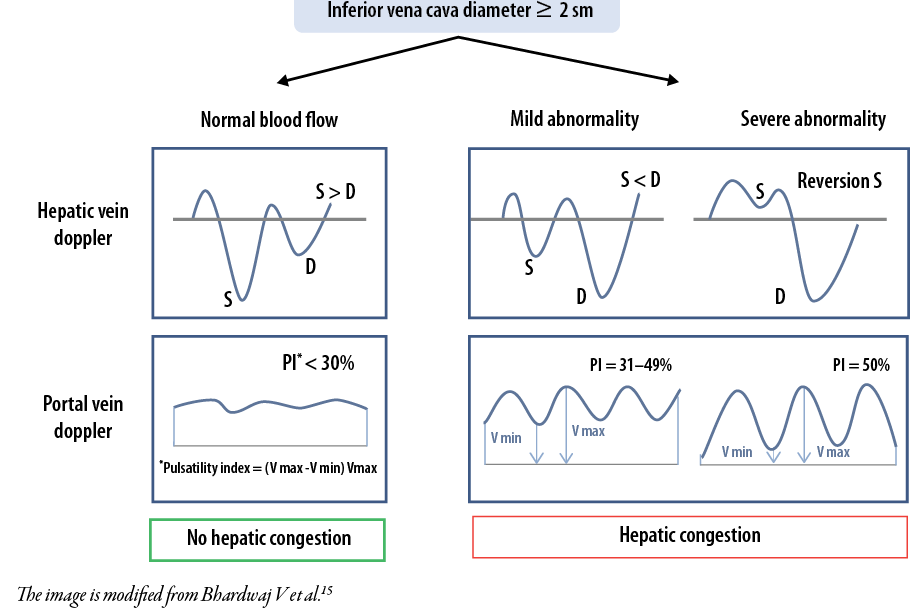 Figure 1: Assessment of hepatic venous congestion using Dopplerography.
Figure 1: Assessment of hepatic venous congestion using Dopplerography.
Assessment of long-term clinical outcomes was carried out by a structured telephone survey 1, 3, 6, and 12 months after discharge. Combined parameters of total mortality and repeated hospitalizations were evaluated as the endpoint.
Analysis was performed using SPSS (IBM SPSS Statistics for Windows, version 22.0; IBM, Armonk, NY, USA). Quantitative variables were described as arithmetic mean±SD (with a normal distribution), or as the median and IQR (with an asymmetric distribution). The threshold survival value for each method was determined from the respective receiver operating characteristic curve. To assess the prognostic significance of different methods for the risk of occurrence of variables of interest, single- and multi-factor models of Cox regression analysis were used. The variables included in the model were selected based on their significance. The probability of survival was estimated by Kaplan-Meyer survival curves and the comparison was made using lograng criterion. Statistical significance was set at p < 0.05.
Results
The participants comprised 207 chronic HF patients (54.1% men, average age = 70.7±12.8 years) who were hospitalized for decompensation of HF. The clinical and demographic characteristics of patients are presented in Table 1. Most (88.9%) participants had a history of arterial hypertension, 49.3% had coronary heart disease, and 33.3% had type 2 diabetes mellitus.
Table 1: Clinical and demographic characteristics of patients (N = 207).
|
Sex
|
|
|
Male, n (%)
|
112 (54.1)
|
|
Female, n (%)
|
95 (45.9)
|
|
Age, mean ± SD, year
|
70.7 ± 12.8
|
|
Systolic BP median; IQR, mmHg
|
117; 106–132
|
|
Diastolic BP median; IQR, mmHg
|
69; 62–77
|
|
LVEF median; IQR, %
|
44.0; 33.0–55.0
|
|
NT-proBNP median; IQR, pg/mL
|
1076; 609–2098
|
|
Arterial hypertension, n (%)
|
184 (88.9)
|
|
Coronary heart disease, n (%)
|
102 (49.3)
|
|
History of myocardial infarction, n (%)
|
67 (32.4)
|
|
Atrial fibrillation, n (%)
|
132 (63.8)
|
IQR: interquartile range (25th and 75th percentile); BP: blood pressure; LVEF: left ventricular ejection fraction; NT-proBNP: N-terminal prohormone of brain natriuretic peptide.
Doppler curves of liver congestion (according to the VExUS protocol) persisted in 57 (27.5%) patients as shown in Table 2 reveals. Table 3 displays the threshold values for predicting outcomes depending on the method.
Table 2: Characteristics of patients depending on the presence of hepatic congestion at discharge (N = 207).
|
Age, mean ± SD, years
|
71.0 ± 12.7
|
70.6 ± 12.9
|
70.7 ± 12.8
|
0.876
|
|
Male, n (%)
|
37 (64.9)
|
75 (50.0)
|
112 (54.1)
|
0.054
|
|
Coronary heart disease, n (%)
|
31 (54.4)
|
71 (47.3)
|
102 (49.3)
|
0.387
|
|
History of myocardial infarction, n (%)
|
19 (33.3)
|
48 (32.0)
|
67 (32.4)
|
0.902
|
|
Arterial hypertension, n (%)
|
47 (82.5)
|
137 (91.3)
|
184 (88.9)
|
0.049*
|
|
Type 2 diabetes mellitus, n (%)
|
16 (28.1)
|
53 (35.3)
|
69 (33.3)
|
0.322
|
|
LVEF median; IQR, %
|
38.0; 30.0–48.5
|
48.0; 35.0–55.0
|
44; 33–55
|
< 0.001*
|
|
NT-proBNP median; IQR, pg/mL
|
2077; 1442–2795
|
923; 452–1641
|
1076; 609–2098
|
< 0.001*
|
|
Liver density median; IQR, kPa
|
15.5; 10.1–25.5
|
5.7; 4.4–7.6
|
6.7; 5.0–12.5
|
< 0.001*
|
*Significance; IQR: interquartile range (25th and 75th percentile); LVEF: left ventricular ejection fraction NT-proBNP: N-terminal prohormone of brain natriuretic peptide..
Table 3: Threshold values for predicting outcomes depending on the method.
|
Ultrasound of lungs, no. of B-lines
|
> 5
|
66.7
|
63.2
|
0.610
|
0.044
|
AUC: area under the curve.
Median follow-up time was 364 days (IQR = 197–365). During this period, 63 (30.4%) events were detected, including 23 deaths (11.1%) [Table 4].
Table 4: Values of congestion markers in patients with acute decompensated heart failure depending on outcomes (N = 207).
|
Lungs ultrasound
(No. of B-lines) median; IQR
|
9; 4–18
|
6.5; 3–13
|
18; 9–26
|
< 0.001
|
|
Liver density median; IQR, kPa
|
6.7; 5.0–12.5
|
6; 4.7–10.2
|
8.3; 5.9–16.0
|
< 0.001
|
|
No. of patients with venous congestion, n (%)
|
57 (27.5)
|
27 (18.8)
|
30 (47.6)
|
< 0.001
|
NT-proBNP: N-terminal prohormone of brain natriuretic peptide.
Univariate Cox regression analysis demonstrated independent prognostic value with the total endpoint of all markers of congestion evaluated by different methods, such as lung ultrasound (HR = 7.0, 95% CI: 2.8 – 17.6; p <0.001), ultrasound evaluation of hepatic venous stagnation according to the VExUS protocol (HR = 2.9, 95% CI: 1.7–4.8; p < 0.001), liver density index according to indirect elastometry (HR = 2.2, 95% CI: 1.3–3.8; p = 0.003). Multivariate Cox regression analysis retained prognostic significance with respect to adverse outcomes for liver density > 6.2 kPa (HR = 1.9, 95% CI: 1.0 – 3.3; p = 0.029) and hepatic venous congestion according to the VExUS protocol (HR = 2.8, 95% CI: 1.3–5.7; p = 0.004).
The Kaplan-Meyer survival curves revealed significant differences in the cumulative probability of survival between groups of patients based on the following parameters: (a) the number of B-lines [Figure 2], (b) liver density [Figure 3], and (c) hepatic venous congestion according to the VExUS protocol [Figure 4].
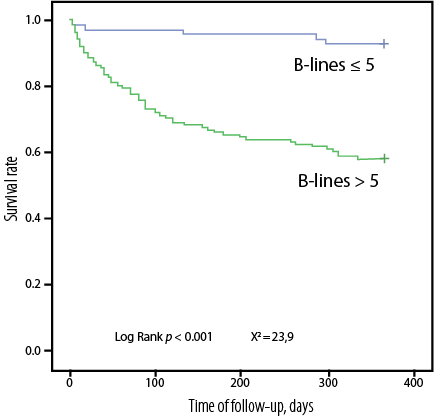 Figure 2: Kaplan-Meyer curves of cumulative probability of survival (total mortality + re-hospitalization) depending on the presence of pulmonary congestion according to ultrasound data.
Figure 2: Kaplan-Meyer curves of cumulative probability of survival (total mortality + re-hospitalization) depending on the presence of pulmonary congestion according to ultrasound data.
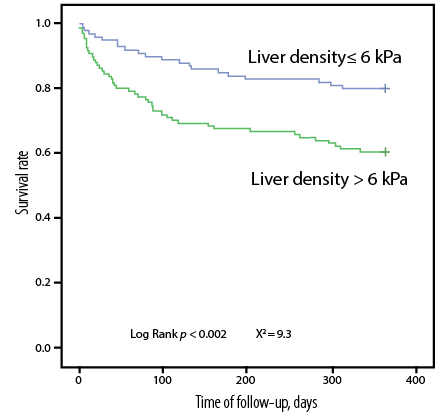 Figure 3: Kaplan-Meyer curves of cumulative probability of survival (total mortality + re-hospitalization) depending on liver density according to indirect elastometry.
Figure 3: Kaplan-Meyer curves of cumulative probability of survival (total mortality + re-hospitalization) depending on liver density according to indirect elastometry.
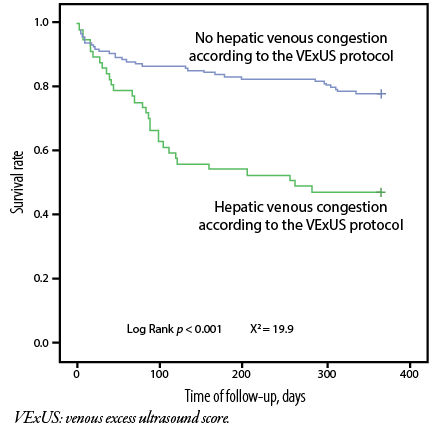 Figure 4: Kaplan-Meyer curves of cumulative survival probability (total mortality + re-hospitalization) depending on the presence of hepatic venous congestion according to the VExUS protocol.
Figure 4: Kaplan-Meyer curves of cumulative survival probability (total mortality + re-hospitalization) depending on the presence of hepatic venous congestion according to the VExUS protocol.
The Kaplan-Meyer curves of cumulative survival probability (total mortality + rehospitalization) given by the different methods used to assess congestion are shown in Figure 5. A significant increase in the risk of prognostic value was found in association with congestion detected by liver lung ultrasound + fibroelastometry (HR = 10.5, 95% CI: 2.3–46.2; p = 0.002), lung ultrasound + ultrasound of hepatic veins (HR = 16.7, 95% CI: 3.9–70.7; p < 0.001), and with all three methods (HR = 40.1, 95% CI: 6.6–243.1; p < 0.001).
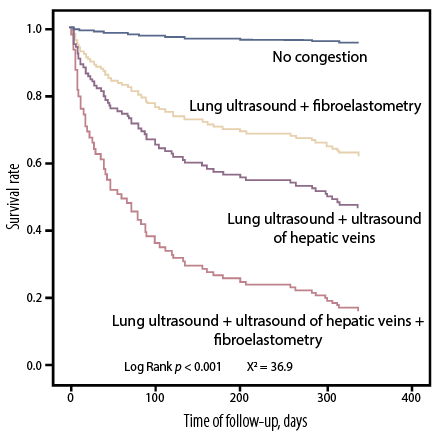 Figure 5: Kaplan-Meyer curves of cumulative survival probability (total mortality+ rehospitalization) depending on the number of methods used to assess congestion.
Figure 5: Kaplan-Meyer curves of cumulative survival probability (total mortality+ rehospitalization) depending on the number of methods used to assess congestion.
Discussion
The major finding of our study is that a comprehensive assessment of congestion at discharge from the hospital can predict adverse events (survival and rehospitalization) in patients with ADHF at one year. We also found that individuals in whom congestion was detected in both blood circulation and by each of the three methods (ultrasound of the lungs, ultrasound of the hepatic veins according to the VExUS protocol, and liver fibroelastometry) had the worst prognosis.
In previous studies, retention of B-lines was associated with increased risk of rehospitalization for congestive HF at 3 and 6 months,16 and patients with liver density > 6.9 kPa were characterized by a higher frequency of death and repeated hospitalizations for HF (HR = 3.57, 95% CI: 1.93–6.83; p < 0.001).17 In other studies, venous congestion according to the VExUS protocol was associated with adverse outcomes including re-hospitalization for congestive HF, cardiovascular mortality, and total mortality at 12-month follow-up.11,18–20
In our study, comprehensive assessment of congestion using a combination of hepatic vein ultrasound + lung ultrasound had the greatest prognostic significance (HR = 16.7, 95% CI: 3.9–70.7; p < 0.001), and not the combination of lung ultrasound + liver fibroelastometry (HR = 10.5, 95% CI: 2.3–46.2; p = 0.002), which, according to the literature, has the greatest prognostic significance.21
Conclusion
In patients with acute decompensated HF, comprehensive assessment of congestion before discharge using ultrasound of the lungs counting the sums of B-lines, assessment of hepatic veins according to the VExUS protocol, and liver fibroelastometry are useful in predicting survival and re-hospitalization at one year.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Harjola VP, Mullens W, Banaszewski M, Bauersachs J, Brunner-La Rocca HP, Chioncel O, et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the acute heart failure committee of the heart failure association (HFA) of the European society of cardiology (ESC). Eur J Heart Fail 2017 Jul;19(7):821-836.
- 2. Fudim M, Hernandez AF, Felker GM. Role of volume redistribution in the congestion of heart failure. J Am Heart Assoc 2017 Aug 17;6(8):e006817.
- 3. Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, et al; European Society of Cardiology; European Society of Intensive Care Medicine. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European society of cardiology and endorsed by the European society of intensive care medicine. Eur J Heart Fail 2010 May;12(5):423-433.
- 4. O’Connor C, Fiuzat M, Mulder H, Coles A, Ahmad T, Ezekowitz JA, et al. Clinical factors related to morbidity and mortality in high-risk heart failure patients: the GUIDE-IT predictive model and risk score. Eur J Heart Fail 2019 Jun;21(6):770-778.
- 5. Wehbe RM, Khan SS, Shah SJ, Ahmad FS. Predicting high-risk patients and high-risk outcomes in heart failure. Heart Fail Clin 2020 Oct;16(4):387-407.
- 6. Kobalava ZhD, Tolkacheva VV, Sarlykov BK, Cabello FE, Bayarsaikhan M, Diane ML, et al. Integral assessment of congestion in patients with acute decompensated heart failure. Russian Journal of Cardiology 2022;27(2):4799.
- 7. Curbelo J, Rodriguez-Cortes P, Aguilera M, Gil-Martinez P, Martín D, Suarez Fernandez C. Comparison between inferior vena cava ultrasound, lung ultrasound, bioelectric impedance analysis, and natriuretic peptides in chronic heart failure. Curr Med Res Opin 2019 Apr;35(4):705-713.
- 8. Omote K, Nagai T, Asakawa N, Kamiya K, Tokuda Y, Aikawa T, et al. Impact of admission liver stiffness on long-term clinical outcomes in patients with acute decompensated heart failure. Heart Vessels 2019 Jun;34(6):984-991.
- 9. Poelzl G, Auer J. Cardiohepatic syndrome. Curr Heart Fail Rep 2015 Feb;12(1):68-78.
- 10. Nikolaou M, Parissis J, Yilmaz MB, Seronde MF, Kivikko M, Laribi S, et al. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur Heart J 2013 Mar;34(10):742-749.
- 11. Beaubien-Souligny W, Rola P, Haycock K, Bouchard J, Lamarche Y, Spiegel R, et al. Quantifying systemic congestion with point-of-care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J 2020 Apr;12(1):16.
- 12. Colom G, Salvador JP, Acosta G, Albericio F, Royo M, Marco MP. Competitive ELISA for N-terminal pro-brain natriuretic peptide (NT-proBNP) determination in human plasma. Analyst 2020 Oct;145(20):6719-6727.
- 13. Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006 Mar;55(3):403-408.
- 14. Rola P, Miralles-Aguiar F, Argaiz E, Beaubien-Souligny W, Haycock K, Karimov T, et al. Clinical applications of the venous excess ultrasound (VExUS) score: conceptual review and case series. Ultrasound J 2021 Jun;13(1):32.
- 15. Bhardwaj V, Vikneswaran G, Rola P, Raju S, Bhat RS, Jayakumar A, et al. Combination of inferior vena cava diameter, hepatic venous flow, and portal vein pulsatility index: venous excess ultrasound score (VEXUS score) in predicting acute kidney injury in patients with cardiorenal syndrome: a prospective cohort study. Indian J Crit Care Med 2020 Sep;24(9):783-789.
- Coiro S, Rossignol P, Ambrosio G, Carluccio E, Alunni G, Murrone A, et al. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur J Heart Fail 2015 Nov;17(11):1172-1181.
- 16. Taniguchi T, Ohtani T, Kioka H, Tsukamoto Y, Onishi T, Nakamoto K, et al. Liver stiffness reflecting right-sided filling pressure can predict adverse outcomes in patients with heart failure. JACC Cardiovasc Imaging 2019 Jun;12(6):955-964.
- 17. Denault A, Couture EJ, De Medicis É, Shim JK, Mazzeffi M, Henderson RA, et al. Perioperative Doppler ultrasound assessment of portal vein flow pulsatility in high-risk cardiac surgery patients: a multicentre prospective cohort study. Br J Anaesth 2022 Nov;129(5):659-669.
- 18. Rajan R, Al Jarallah M. New prognostic risk calculator for heart failure. Oman Med J 2018 May;33(3):266-267.
- 19. Al-Jarallah M, Rajan R, Al-Zakwani I, Dashti R, Bulbanat B, Ridha M, et al. Mortality and morbidity in HFrEF, HFmrEF, and HFpEF patients with diabetes in the Middle East. Oman Med J 2020 Jan;35(1):e99.
- 20. Kobalava Z, Tolkacheva V, Cabello Montoya F, Sarlykov B, Al-Jarallah M, Brady P, et al. Prognostic value of integral assessment of congestion in patients hospitalized with acute decompensated chronic heart failure: a single center study. Annals of Clinical Cardiology 2022;4(2):77-84.