Meningiomas account for almost 38% of all primary central nervous system tumors and nearly 50% of all benign brain tumors.1 Meningiomas develop in the meningeal layers of the brain or spinal cord with a female-to-male ratio of 2:3 and 66 years as the average age of presentation.1,2 The World Health Organization (WHO) 2021 criteria classifies meningiomas into three grades.3 The vast majority (80–81%) of meningiomas are categorized as grade 1 (typical), while 17–18% are classified as grade 2 (atypical), and 1.7% as grade 3 (anaplastic).1,2 Magnetic resonance imaging sequences are useful to diagnose and evaluate brain tumors, but ineffective in differentiating between their types or grades.4
Meningiomas have an inherent risk of recurrence, despite apparently complete surgical removal.5,6 The mean 10-year recurrence rate of an excised meningioma can reach 20%.7 The higher the meningioma grade, the more likely the recurrence. Recurrence rates between 50% and 94% have been reported for grade 3 meningiomas, 29–52% for grade 2, and 7–25% for grade 1.8 Apart from the known risk factors such as histological malignancy grade and subtotal resection, other factors such as young age, specific histological subtypes, and high proliferative rate may also influence the probability of recurrence.9
A high mitotic index is considered to be a strong indicator of tumor recurrence.10 The molecular immunology borstel-1 (MIB-1) monoclonal antibody has been used frequently to stain Ki-67 antigen, to investigate the growth index of various systemic and intracranial neoplasms. In astrocytic tumors, the monoclonal antibody Ki-67/MIB-1 is frequently employed and has demonstrated prognostic and diagnostic power.11 A nuclear antigen called Ki-67 is expressed in the cell cycle’s G1, S, G2, and M stages. This nuclear antigen is expressed by proliferating cells throughout the cell cycle, and the monoclonal antibody MIB-1 can identify it. The MIB-1 labeling index (LI) is a measure of the proportion of immunopositive cells in a tumor.11,12 Meningiomas with indices > 4% have an increased risk of recurrence similar to grade 2 (atypical) meningioma, whereas those with indices > 20% are associated with death rates analogous to those with grade 3 (anaplastic) meningioma.13 High Ki-67/MIB-1 LI is associated with a high meningioma recurrence rate.14
The relation between sex hormone receptors and meningiomas has been the subject of several studies.15–18 The higher incidence of meningiomas in women, their growth during pregnancy and luteal phase with a subsequent decrease after delivery, and their association with breast carcinoma suggest that this type of tumor could be hormone-dependent.19 Expression of progesterone receptor (PR) by meningioma cells is prognostically a favorable sign, while the absence of PR expression would be accompanied by a more aggressive tumoral behavior.20 Thus, the expression of PRs may relate to tumor grade and recurrence. There is a significant correlation between negative PR status and high MIB-1 LI.21 The absence of PR, high mitotic index, and tumor grade are significant factors in assessing disease-free survival.22 Studies on the function of different prognostic indicators in meningiomas are very few. Hence, this study was undertaken to determine the immunohistochemistry expression of hormone receptors and proliferation markers in meningiomas and correlating them with the grade of meningioma and its recurrence.
Methods
This collaborative study was conducted in the department of pathology and neurosurgery of a tertiary care center in the state of Jammu and Kashmir, India. A total of 276 cases for 10 years from December 2012 to 2022 (retrospective data for 8.5 years and prospective cases for 1.5 years) were included in the study. Relevant paraffin-embedded tissue blocks were taken out. From each block, four 3-micron thick sections were taken. One section was stained with hematoxylin and eosin for revision of the histopathological diagnosis, and the remaining three were stained immunohistochemically. A heat-induced approach using a pressure cooker and Tris ethylenediaminetetraacetic acid at pH 9 was used for antigen retrieval. Hydrogen peroxide at 3% inhibited endogenous peroxidase activity. The sections were cleaned in Tris buffer (pH 7.6) and then incubated for 30 min at room temperature with rabbit monoclonal primary antibodies against estrogen receptor (ER) (clone SP1, Ventana) and PR (clone 1E2, Ventana). With interim washes in Tris buffer (pH 7.6), the slides were treated with poly-horseradish peroxidase reagent and diaminobenzidine chromogen. ER- and PR-positive invasive ductal carcinoma of the breast was utilized as positive control. The tumor cells with nuclear ER and PR staining were regarded as positive. For MIB-1, monoclonal mouse anti-human Ki-67 antigen, clone M 7240 (Dako, Denmark) was used. Technical negative control from a lymph node with follicular lymphoid hyperplasia known to be immunoreactive for Ki-67 was used for MIB-1.
The stained slides were examined by two pathologists and the tumors were classified into subtypes according to the dominant growth pattern (roughly 50% of a specimen on microscopic evaluation) on hematoxylin and eosin-stained sections. The previously recorded grades were evaluated and updated where necessary (though the need for change was minimal) as per the WHO 2021 classification of central nervous system tumors.2
Immunohistochemical evaluation was conducted on a total of 75 meningioma cases with the following grade-wise breakup: all 34 grade 2 cases, all 11 grade 3 cases, and 30 of 231 (13%; randomly selected) grade 1 cases of meningioma. The MIB-1 LI was determined using the following semi-quantitative scale: 0: absent, 1: weak, 2: moderate, and 3: strong. The percentage of positive tumor cells in a section was graded as follows: 0: 0%, 1: < 10%, 2: 10–50%, 3: 51–80%, and 4: > 80% positive tumor nuclei. An immunoreactive score (IRS) ranging from 0–12 was determined for each tumor by the recommendations for breast cancer and meningioma tissues. The IRS was computed by multiplying the staining intensity by the indicator for positive tumor cells. Cancers with an IRS ≥ 2 were deemed to be receptor-positive as described by Roser et al.22
The data was analyzed using SPSS Statistics (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp). The values were measured as median, mean, and SD according to the considered variables. The chi-square test was used to assess the association of sex, grade, and histological subtype of meningioma. P-value < 0.05 was taken as significant.
Results

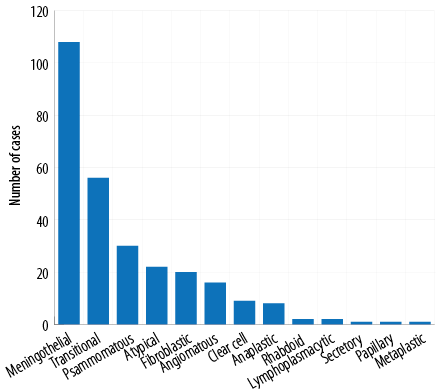 Figure 1: Histological spectrum of meningiomas.
Figure 1: Histological spectrum of meningiomas.

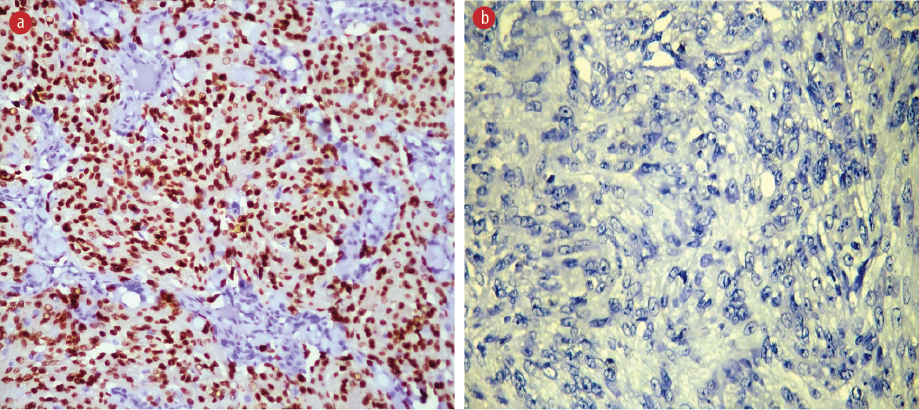 Figure 2: Immunohistochemical slides showing progesterone receptor expression in (a) grade 1 and (b) grade 3 meningioma, magnification = 40 ×.
Figure 2: Immunohistochemical slides showing progesterone receptor expression in (a) grade 1 and (b) grade 3 meningioma, magnification = 40 ×.
Table 1: The prevalence of WHO 2021 meningioma grades compared with immunohistochemical prevalence in each grade (N = 276).
|
1
|
71
|
160
|
231
|
26.0
|
70.0
|
2.1
|
|
2
|
22
|
12
|
34
|
5.8
|
20.0
|
6.3
|
WHO: World Health Organization; MIB-1 LI: molecular immunology borstel-1 labeling index.

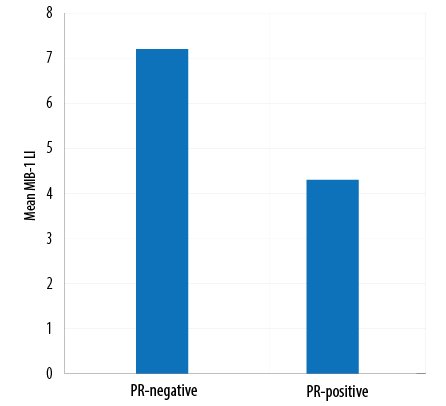 Figure 3: Mean MIB-1 labeling index (LI) versus progesterone receptor (PR) status.
Figure 3: Mean MIB-1 labeling index (LI) versus progesterone receptor (PR) status.

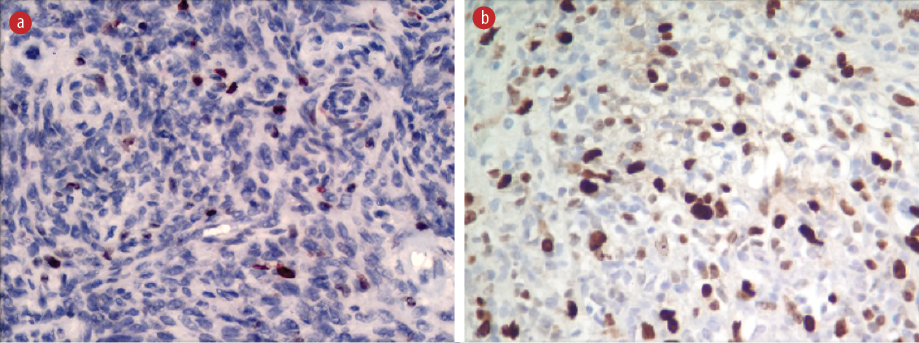 Figure 4: Molecular immunology borstel-1 immunostaining with the regional variability of labeling index between (a) grade 2 meningioma and (b) grade 3 meningioma, magnification = 40 ×.
Figure 4: Molecular immunology borstel-1 immunostaining with the regional variability of labeling index between (a) grade 2 meningioma and (b) grade 3 meningioma, magnification = 40 ×.
A total of 276 patients (all having Kashmiri ethnicity) were included in our study. Their mean age was 49.7 years (range = 5–73 years). The maximum number of patients was in the 40–60 age group and five (1.8%) were < 18 years old. Most (179; 64.9%) patients were female, giving a female: male ratio of 1.8:1. Meningothelial meningioma was the most common histological type (38.8%) followed by transitional meningiomas (19.6%) and others [Figure 1].
WHO grade 1 meningioma was present in 231 (83.7%) patients, grade 2 in 34 (12.3%) patients, and grade 3 in 11 (4.0%) patients. The majority (69.3%) of grade 1 meningiomas occurred in females. However, grades 2 and 3 meningiomas were more common in males (64.7% of grade 2 and 54.5% of grade 3).
Immunohistochemical evaluation for the expressions of ER, PR, and MIB-1 LI was conducted in 30 cases of grade 1, 34 cases of grade 2, and 11 cases of grade 3 meningioma. As the grade of meningiomas increased, the percentage positivity for ER and PR decreased significantly [Figures 2 and 3].
The MIB-1 LI in our samples ranged from 0.0–35.0%, with statistically significant differences between grades. While correlating the PR-expression with the MIB-1 LI, it was observed that mean MIB-1 LI was higher in PR-negative cases (7.2%) than in PR-positive cases (4.3%), showing an inverse correlation [Figure 4]. The mean MIB-1 LI was higher in males (7.2%) than in females (4.5%).
In our study, there were 12 (4.3%) cases of recurrent meningiomas (seven males and five females). The mean age of recurrence was 49.0±13.5 years (range = 24–65 years). Six (50.0%) of these patients were < 40 and the other six were > 65. Out of the 12 recurrent cases, only three expressed PR and one case was ER-positive [Table 1].
Among the recurrent cases, it was seen that grade 2 tumors did not change their grade on recurrence. However, five out of eight grade 1 tumors recurred as grade 2. The mean time for recurrence to develop was 8.3 years (range = 4 months–32 years). The median MIB-1 LI of recurrent cases was 16.7, which was significantly higher than that of non-recurrent cases.
Discussion
The majority of our patients were female and this was similar to the sex ratios in studies by Shayanfar et al,23 and Al-Nuaimy et al.24 Grade 1 tumors formed the majority of our cases (83.7%), followed by grade 2 (12.3%) and grade 3 tumors (4.0%). Our results were in concordance with most studies in the literature with grade 1 meningiomas showing a female preponderance, whereas grade 2 and grade 3 meningiomas were more common in males, 64.7% and 4.5%, respectively.
In our study, the median MIB-1 LI for grade 1 was 2.1, for grade 2 was 6.3, and for grade 3 meningiomas was 13.4. Shayanfar et al,23 recorded a mean MIB-1 LI of 2.98±2.27 in grade 1 tumors, 9.30±5.79 in grade 2 tumors, and 34.00±5.47 in grade 3 tumors. Studies by Mukhopadhyay et al,25 and Dutta et al,26 yielded similar results.
In our grade 1 meningiomas patients, MIB-1 LI functioned as a control for comparison with higher-grade meningiomas and was useful in cases with some but not all characteristics of atypical meningioma. We also noted a few benign grade 1 meningiomas with higher-than-expected MIB-1 LI (> 7%) suggesting the possibility that the biopsies may not have included the focal areas of histological atypia.
Al-Nuaimy et al,24 documented mean Ki-67 LI to be higher in the histological types of grade 3 and grade 2 meningiomas with a mean Ki-67 LI±SD of 13±13% in anaplastic meningioma and mean Ki-67 LI±SD of 5.4±2.8% in atypical meningiomas. Kolles et al,27 found Ki-67 (MIB-1) LI to be the most important criterion for distinguishing grade 3 meningiomas (mean Ki-67 LI = 11%) from grade 1 (mean Ki-67 LI = 0.7%). Akyildiz et al,28 found a significant relationship between Ki-67 LI and mitotic activity, necrosis, pattern loss, small cell changes, and brain invasion. Thus, our results are in concordance with several studies in the literature.
No correlation between MIB-1 LI and the histology of meningiomas was observed in our study, although Özen et al,29 reported the highest MIB-1 LI value in fibrous and lowest in secretory meningiomas.
In the current study, the mean MIB-1 LI was higher in males (7.16%) than in females (4.5%). A similarly significant influence of sex was found by Al-Nuaimy et al.24
Quantifying the hormonal status of a tumor may help predict its biological behavior and provide options for further treatments. The higher incidence of meningiomas among women has led to the assumption that steroid sex hormones may influence their growth.30
ER expression was observed in 13.3% of our cases. It was seen that 26.0% of grade 1, 5.8% of grade 2, and none of grade 3 meningiomas expressed the ER (p < 0.019). No significant correlation of estrogen expression was observed in our study with histological subtype or sex. Fakhrjou et al,31 observed ER expression in 20% of cases and Dutta et al,26 observed 20.89%. Our results were in concordance with several studies, which reported low or absent expression of ER in meningiomas.
In our study, 70.0% of grade 1, 20.0% of grade 2, and 18.0% of grade 3 meningiomas expressed PRs. Comparable results were observed by Roser et al,30 Al-Nuaimy et al,24 and Dutta et al.26
Although not entirely unlikely, no association between PR status and age, sex, tumor location, and histology were found in the literature.18,30 Statistical analysis of our data was done to confirm this, and we found that no significant relation of PR expression was seen in the above-mentioned variables. However, many studies in the literature point to higher expression of PR in female patients.22,32 However, these studies included a substantial number of atypical and malignant meningiomas. Therefore, the previously reported sex-related differences might be due to differences in selection criteria, which may have produced non-homogeneous patient populations.
The mean MIB-1 LI among our participants was higher in PR-negative cases (7.2%) than in PR-positive cases (4.3%), showing an inverse correlation. A similar trend was reported by Al-Nuaimy et al.24
We documented 12 (4.3%) cases of recurrent meningiomas with a male to female ratio of 1.4:1 — too few cases to derive any statistically significant pattern. The mean age of recurrence was 49.0±13.5 years and the mean period of recurrence was 8.3 years. Other studies also have reported higher recurrence rates for males than for females.6,33 Among recurring meningioma cases, 66.7% were grade 2, 25.0% were grade 1, and 8.3% were grade 3. In our study, grade 2 tumors did not change their grade on recurrence. However, some of grade 1 meningiomas became grade 2. Only one out of the 12 cases showed expression of ER. Half of grade 1, 16.0% of grade 2, and none of grade 3 meningiomas were positive for PRs expression. In this respect, our findings are similar to those elsewhere. PR negativity was strongly correlated with the recurrence of benign meningiomas, whereas the ER status was not significant.32 Roser et al,30 suggested that PR status alone could not be used to predict prognosis. Instead, in combination with the proliferative index, it could be a useful prognostic tool for benign meningiomas. Similar to findings elsewhere,34 the mean MIB-1 LI in our recurrent cases was higher than that of nonrecurrent cases. Hence our results, like those of other studies, found that higher MIB-1 LI represented a higher risk of recurrence, with the caveat that cut-off levels and counting techniques have varied considerably between those studies.
The limitation of this study is that it was conducted at a single center located in Srinagar, India, which caters to the local population of mostly Kashmiri ethnicity. This diminishes the generalizability of its findings.
Conclusion
The grades and recurrence of meningiomas have significant positive relationships with MIB-1 LI. For routine examination of meningiomas, especially those with borderline atypia, MIB-1 LI is a helpful auxiliary approach. However, PR status has a strong correlation with the histological grade, and its expression is a positive prognostic indicator. When combined with MIB-1 LI, PR status can shed more light on the behavior of a meningioma, especially when subtotal resection, a high proliferative rate, or recurrence are present. This information can also be used as a prognostic tool to forecast a tumor’s recurrence. Because meningiomas take a long time to recur, long-term follow-up investigations are recommended.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol 2019 Nov;21(Suppl 5):v1-v100.
- 2. Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, Lukas RV. An overview of meningiomas. Future Oncol 2018 Sep;14(21):2161-2177.
- 3. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 2021 Aug;23(8):1231-1251.
- 4. Momeni F, Abedi-Firouzjah R, Farshidfar Z, Taleinezhad N, Ansari L, Razmkon A, et al. Differentiating between low- and high-grade glioma tumors measuring apparent diffusion coefficient values in various regions of the brain. Oman Med J 2021 Mar;36(2):e251.
- 5. Maiuri F, De Caro MB, Esposito F, Cappabianca P, Strazzullo V, Pettinato G, et al. Recurrences of meningiomas: predictive value of pathological features and hormonal and growth factors. J Neurooncol 2007 Mar;82(1):63-68.
- 6. Corniola MV, Meling TR. Management of recurrent meningiomas: state of the art and perspectives. Cancers (Basel) 2022 Aug;14(16):3995.
- 7. Varlotto J, Flickinger J, Pavelic MT, Specht CS, Sheehan JM, Timek DT, et al. Distinguishing grade I meningioma from higher grade meningiomas without biopsy. Oncotarget 2015 Nov;6(35):38421-38428.
- 8. Backer-Grøndahl T, Moen BH, Torp SH. The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol 2012;5(3):231-242.
- 9. Ogasawara C, Philbrick BD, Adamson DC. Meningioma: a review of epidemiology, pathology, diagnosis, treatment, and future directions. Biomedicines 2021 Mar;9(3):319.
- 10. Olar A, Wani KM, Sulman EP, Mansouri A, Zadeh G, Wilson CD, et al. Mitotic index is an independent predictor of recurrence-free survival in Meningioma. Brain Pathol 2015 May;25(3):266-275.
- 11. Chaloob MK, Ali HH, Qasim BJ, Mohammed AS. Immunohistochemical expression of Ki-67, PCNA and CD34 in astrocytomas: a clinicopathological study. Oman Med J 2012 Sep;27(5):368-374.
- 12. Gözü H, Bilgiç B, Hazneci J, Sargın H, Erkal F, Sargın M, et al. Is Ki-67 index a useful labeling marker for invasion of pituitary adenomas? Turk J Endocrinol Metab 2005;4:107-113.
- 13. Wilson TA, Huang L, Ramanathan D, Lopez-Gonzalez M, Pillai P, De Los Reyes K, et al. Review of atypical and anaplastic meningiomas: classification, molecular biology, and management. Front Oncol 2020 Nov;10:565582.
- 14. Abry E, Thomassen IØ, Salvesen ØO, Torp SH. The significance of Ki-67/MIB-1 labeling index in human meningiomas: a literature study. Pathol Res Pract 2010 Dec;206(12):810-815.
- 15. Strik HM, Strobelt I, Pietsch-Breitfeld B, Iglesias-Rozas JR, Will B, Meyermann R. The impact of progesterone receptor expression on relapse in the long-term clinical course of 93 benign meningiomas. In Vivo 2002;16(4):265-270.
- 16. Kane AJ, Sughrue ME, Rutkowski MJ, Shangari G, Fang S, McDermott MW, et al. Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer 2011 Mar;117(6):1272-1278.
- 17. Pravdenkova S, Al-Mefty O, Sawyer J, Husain M. Progesterone and estrogen receptors: opposing prognostic indicators in meningiomas. J Neurosurg 2006 Aug;105(2):163-173.
- 18. Agopiantz M, Carnot M, Denis C, Martin E, Gauchotte G. Hormone receptor expression in meningiomas: a systematic review. Cancers (Basel) 2023 Feb;15(3):980.
- 19. Hortobágyi T, Bencze J, Murnyák B, Kouhsari MC, Bognár L, Marko-Varga G. Pathophysiology of meningioma growth in pregnancy. Open Med (Wars) 2017 Jul;12(1):195-200.
- 20. Chargari C, Védrine L, Bauduceau O, Le Moulec S, Ceccaldi B, Magné N. Reapprasial of the role of endocrine therapy in meningioma management. Endocr Relat Cancer 2008 Dec;15(4):931-941.
- 21. Perry A, Cai DX, Scheithauer BW, Swanson PE, Lohse CM, Newsham IF, et al. Merlin, DAL-1, and progesterone receptor expression in clinicopathologic subsets of meningioma: a correlative immunohistochemical study of 175 cases. J Neuropathol Exp Neurol 2000 Oct;59(10):872-879.
- 22. Roser F, Nakamura M, Bellinzona M, Rosahl SK, Ostertag H, Samii M. The prognostic value of progesterone receptor status in meningiomas. J Clin Pathol 2004 Oct;57(10):1033-1037.
- 23. Shayanfar N, Mashayekh M, Mohammadpour M. Expression of progestrone receptor and proliferative marker ki 67 in various grades of meningioma. Acta Med Iran 2010;48(3):142-147.
- 24. Al-Nuaimy WM, Jalal JA, Mohammed BB. Ki-67(MIB-1) and progesterone receptor in meningioma: an immunohistochemical study. Iraqi Postgrad Med J 2012;11(2):157-167.
- 25. Mukhopadhyay M, Das C, Kumari M, Sen A, Mukhopadhyay B, Mukhopadhyay B. Spectrum of meningioma with special reference to prognostic utility of ER,PR and Ki67 expression. J Lab Physicians 2017;9(4):308-313.
- 26. Dutta V, Malik A, Topgay T, Deb P. Immunohistochemical study characterizing estrogen and progesterone receptors status in meningiomas and correlation with MIB-1labeling index. Indian Journal of Pathology: Research and Practice 2012;1(2):53-108.
- 27. Kolles H, Niedermayer I, Schmitt C, Henn W, Feld R, Steudel WI, et al. Triple approach for diagnosis and grading of meningiomas: histology, morphometry of Ki-67/Feulgen stainings, and cytogenetics. Acta Neurochir (Wien) 1995;137(3-4):174-181.
- 28. Akyildiz EU, Oz B, Comunoglu N, Aki H. The relationship between histomorphological characteristics and Ki-67 proliferation index in meningiomas. Bratisl Lek Listy 2010;111(9):505-509.
- 29. Özen O, Demirhan B, Altinörs N. Correlation between histological grade and MIB-1 and p53 immunoreactivity in meningiomas. Clin Neuropathol 2005;24(5):219-224.
- 30. Roser F, Samii M, Ostertag H, Bellinzona M. The Ki-67 proliferation antigen in meningiomas. Experience in 600 cases. Acta Neurochir (Wien) 2004 Jan;146(1):37-44.
- 31. Fakhrjou A, Meshkini A, Shadrvan S. Status of Ki-67, estrogen and progesterone receptors in various subtypes of intracranial meningiomas. Pak J Biol Sci 2012 Jun;15(11):530-535.
- 32. Maiuri F, Mariniello G, de Divitiis O, Esposito F, Guadagno E, Teodonno G, et al. Progesterone receptor expression in meningiomas: Pathological and prognostic implications. Front Oncol 2021 Jul;11:611218.
- 33. Hortobágyi T, Bencze J, Varkoly G, Kouhsari MC, Klekner Á. Meningioma recurrence. Open Med (Wars) 2016 Jun;11(1):168-173.
- 34. Ho DM, Hsu CY, Ting LT, Chiang H. Histopathology and MIB-1 labeling index predicted recurrence of meningiomas: a proposal of diagnostic criteria for patients with atypical meningioma. Cancer 2002 Mar;94(5):1538-1547.