Isolated elephantiasis of the vulva refers to a gigantic swelling of the vulva without concomitant swelling of the lower limbs. Genital elephantiasis is a common result of lymphatic obstruction caused by filariasis, lymphogranuloma venereum (LGV), granuloma inguinale, carcinomas, lymph node dissection, irradiation, or tuberculosis (TB).1 It is a rarely reported entity and its occurrence during pregnancy has been reported only once.2 This likely happens because the primary insult (infection, radiation, or surgery at the vulva, etc.) resolves; however, lymph node or lymphatic vessel damage obstructing to lymph drainage of the vulva persists, which gradually causes lymphoedema and subsequent swelling of the vulva. We report a case of an isolated vulval elephantiasis during pregnancy and we discuss the possible etiologies and management issues. The patient had a successful vaginal delivery followed by a satisfactory genital reconstruction at eight months postpartum with no recurrence thereafter.
Case Report
A 20-year-old primigravida at 22 weeks of gestation presented to the gynecology outpatient wing with the complaint of a large mass at the vulva. This vulval mass was very small before pregnancy but gradually increased in size as her pregnancy progressed. There was no history suggestive of TB, chyluria, swelling of thighs, legs, or feet, genital ulceration, bubo formation, multiple sexual partners, partner with multiple sexual contacts, similar lesion at any other site, or any similar lesion in her partner.
The general physical examination results were unremarkable. The patient’s body mass index was 23 kg/m2, blood pressure was 110/80 mmHg, and pulse rate was 84 per minute. There was no evidence of cervical, axillary, or inguinal lymphadenopathy or features of micronutrient deficiency. Her abdomen was soft and non-tender, and her uterus size on palpation corresponded to 22 weeks’ gestation. The bilateral lower limb appeared normal with no swelling, edema, or ulceration. Local examination of the perineal-vulval region revealed a 12 × 10 cm fleshy, non-tender growth with an irregular surface arising from both labia, merging centrally, and involving the clitoris. There was no involvement of urethral or perianal region [Figure 1]. Gynecological examination revealed a healthy vagina and cervix on speculum examination. On per-vaginal bimanual examination, the cervix was normal, and a gravid uterus of size approximating 22 weeks was palpated.

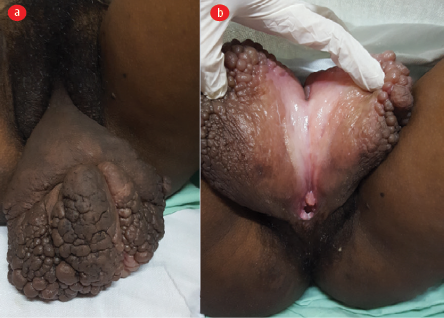 Figure 1: (a) Large mass arising out of and involving bilateral labia minora causing vulval elephantiasis, labia majora appear uninvolved;
Figure 1: (a) Large mass arising out of and involving bilateral labia minora causing vulval elephantiasis, labia majora appear uninvolved;
(b) the same mass lifted and opened depicting the normal vaginal orifice underneath.

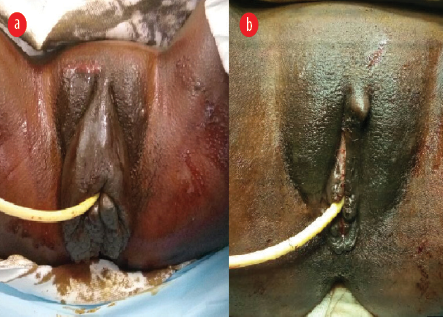 Figure 2: (a) Preoperative photograph depicting regression in the mass size, without complete resolution. (b) Immediate postoperative photograph following reconstruction surgery depicting normal vulval anatomy.
Figure 2: (a) Preoperative photograph depicting regression in the mass size, without complete resolution. (b) Immediate postoperative photograph following reconstruction surgery depicting normal vulval anatomy.
The preliminary blood investigations including hemogram and renal and liver function tests were normal. Tests for sexually transmitted infections including HIV, LGV, genital warts, donovanosis, and syphilis were negative. TB was ruled out on TB-interferon gamma release assay. Microscopic analysis of blood drawn at midnight was performed on three consecutive nights and no microfilariae were found. Antenatal investigations including thyroid function test, hemoglobin electrophoresis, glucose tolerance test, and level II sonography yielded normal results. The mass was biopsied and sent for histopathological examination which reported fragments of fibrocollagenous tissue lined by stratified squamous epithelium. There were no acid-fast bacilli to suggest TB. There was no evidence of any dysplasia.
A provisional diagnosis of vulval elephantiasis of an unknown etiology was made. A meeting was held with the woman and her partner for discussion of the plan for pregnancy and delivery. Anticipated complications and management during the antenatal period, labor, and delivery were discussed. She opted for expectant management of the mass with close observation till delivery and reconstructive surgery later in the postpartum period.
There were no obstetric complications in the remainder of her pregnancy. The vulval mass remained static in size on follow-up. Spontaneous labor and leakage settled in at 37 weeks of gestation, intravenous labor analgesia was provided and her labor progressed uneventfully. A right mediolateral episiotomy, 45° from the midline was given in the second stage of labor after lifting the labial mass. Vaginal delivery yielded a healthy baby boy weighing 2.7 kg with Apgar scores of 8 and 9 at 1 and 5 mins, respectively. There were no maternal injuries, and the episiotomy was repaired in layers.
At six weeks and six months postpartum, she presented with a slight reduction in the size of elephantiasis. Eight months postpartum, the vulval mass was excised and vulval reconstruction was performed under regional anesthesia in liaison with the department of plastic surgery [Figure 2]. The final histopathology report confirmed the mass to be a soft tissue growth lined by stratified squamous epithelium with underlying fibrocollagenous stroma containing dilated lymphatics with no acid-fast bacilli or dysplasia. In the third postoperative year, the patient had an uneventful second pregnancy vaginally delivering a healthy baby at term. There was no recurrence of the vulval mass in the second pregnancy or during the follow-up over two more years.
Discussion
This case of isolated vulval elephantiasis was first brought to medical attention during pregnancy, and etiology could not be ascertained. There have been a few cases of non-pregnant women with such isolated vulval elephantiasis reported in the literature where no etiology could be found. Most cases where etiology could be elucidated were secondary to filariasis, an infection common in tropical countries. Filarial worms (Wuchereria bancrofti and Brugia malayi) lodge in the lymphatic system causing blockage of lymphatic drainage. Filariasis is diagnosed by the presence of microfilariae on blood films or by demonstration of positivity to circulating filarial antigen.3,4 In our case, both tests (blood smears and circulating filarial antigen) were negative, ruling out filariasis.
In the only published case where vulval elephantiasis was concurrent with pregnancy, the cause was active filariasis.2 Cases of vulval elephantiasis associated with lymph node tissue destruction by TB, vulval radiation, or surgery have also been reported.5,6 Some sexually transmitted infections that cause mass lesions at the vulva may appear like lymphatic elephantiasis to the untrained eye such as LGV, genital warts, and granuloma inguinale.7 In our case, all these conditions were excluded. Neoplasm was also ruled out by histopathological examination of the mass.
Further conclusive evidence of lymphatic obstruction could be obtained by performing lymphoscintigraphy; however, our patient refused it due to concerns about fetal exposure to nuclear compounds.8 Thus, to our knowledge, this is the first reported case of idiopathic vulval elephantiasis in a pregnant woman.
Our patient opted for conservative management for vulval elephantiasis to avoid surgery and anesthesia during pregnancy.9 In a previously reported case of vulval elephantiasis in pregnancy, excision of the vulval lesion was performed at five weeks of gestation; however, lymphedema recurred to preoperative proportions by 12 weeks of gestation.2
Many cases of successful surgical removal of the mass with genital reconstruction have been reported among non-pregnant women with such large vulval masses to improve cosmesis and reduce chances of secondary infection due to pressure ulcerations.5,10
This case suggests that successful vaginal delivery is possible in cases with gigantic vulval elephantiasis during pregnancy depending on the size and location of the mass. It may be advisable to postpone the excision of such vulval mass to postpartum period to avoid risks of abortion, recurrence, and anesthetic exposure to the fetus.3
Disclosure
The authors declared no conflicts of interest. Written informed consent was taken from the patient.
Acknowledgments
We thank our patient and her husband for their support.
references
- 1. Kaur M, Malik R, Dutta K, Khera K. A rare case report of large bilateral vulval elephantiasis. Int J Reprod Contracept Obstet Gynecol 2020;9:2642-2645.
- 2. Dorairajan G, Subbaiah MS, Shanbhag ER. Management of vulvar lymphedema of filarial origin in a pregnant woman and its outcome: a case report. J Obstet Gynaecol Res 2019 May;45(5):1076-1078.
- 3. Chakraborty M, Banu H, Chakraborty PP. Bilateral vulval filarial elephantiasis. BMJ Case Rep 2018 Feb;2018:bcr2018224250.
- 4. Mohan H, Bisht B, Goel P, Garg G. Vulval elephantiasis: a case report. Case Rep Infect Dis 2012;2012:430745.
- 5. Arakeri SU, Sinkar P. An unusual gross appearance of vulval tuberculosis masquerading as tumor. Case Rep Obstet Gynecol 2014;2014:815401.
- 6. Ganchua SKC, White AG, Klein EC, Flynn JL. Lymph nodes-The neglected battlefield in tuberculosis. PLoS Pathog 2020;16(8):e1008632.
- 7. Gupta S, Ajith C, Kanwar AJ, Sehgal VN, Kumar B, Mete U. Genital elephantiasis and sexually transmitted infections - revisited. Int J STD AIDS 2006 Mar;17(3):157-165.
- 8. Keleher A, Wendt R III, Delpassand E, Stachowiak AM, Kuerer HM. The safety of lymphatic mapping in pregnant breast cancer patients using Tc-99m sulfur colloid. Breast J 2004;10(6):492-495.
- 9. Ravindra GL, Madamangalam AS, Seetharamaiah S. Anaesthesia for non-obstetric surgery in obstetric patients. Indian J Anaesth 2018 Sep;62(9):710-716.
- 10. Ranjan SK, Sinha M, Jha NK, Sinha DK, Sharan A. Giant vulvar elephantiasis of filarial origin: a rare case report. Journal of Dental and Medical Sciences 2015;14(11):38-40.