One of the most common health conditions affecting modern young men and women is infertility, defined as the failure to achieve a pregnancy after 12 months or more of regular unprotected sexual intercourse.1 Primary infertility is the inability to conceive despite attempts at conception for more than one year, whereas secondary infertility is the inability to become pregnant following a previous successful conception.1 In the past, sexually transmitted infections used to be the leading causes of infertility, but today, stress and lifestyle factors dominate.2
Infertility prevalence has been reported to have been increasing since 1998. According to the World Health Organization, about 186 million individuals and 48 million couples experience fertility problems worldwide with associated medical, economic, and psychological implications including stress and trauma, particularly in Africa, where there is a strong emphasis on childbearing.3 Worldwide, about 11% of women are reported to experience fertility problems against 9% of men, and the impact of advancing age is much greater in women.4,5
The global age-standardized prevalence of female infertility increased from 1367 per 100 000 (1.4%) in 1990 to 1571 per 100 000 (1.6%) in 2017, representing a rise of 14.9% over 18 years. The prevalence of infertility is high in Sub-Saharan Africa, South Asia, the Middle East and North Africa, Central Asia, and Central Europe.6,7 Female infertility can result from a variety of conditions that affect the ovaries, uterus, fallopian tubes, and endocrine system, among others. Recently, oxidative stress has been recognized as a major mediator, especially in polycystic ovary syndrome, endometriosis, and unexplained infertility.7
Fertility assessment methods include ovulation test, ovarian reserve tests, other hormone tests, genetic tests, imaging tests such as hysterosalpingography (HSG), pelvic ultrasound, sonohysterography, and hysteroscopy. Sometimes laparoscopy is used to identify irregularities or blockages of the fallopian tubes, scarring, endometriosis, and problems with the ovaries and uterus. HSG is still the first-line technique to diagnose tubal and intrauterine problems associated with infertility.8
HSG is a non-invasive and simple procedure where a dye that is opaque to X-rays is introduced vaginally into the uterus, which then spreads to the fallopian tubes. An X-ray is taken to visualize the uterine cavity contour and lesions, revealing anomalies in the uterus and the fallopian tubes.9 Despite potential side effects of HSG—such as nausea, fever, pelvic infection, pelvic cramps/pain, lymphogranuloma formation, vasovagal symptoms, radiation exposure, and high probability of false positives—it remains a popular diagnostic tool in the developed and developing regions of the world.9,10
Studies elsewhere have reported the common HSG findings related to infertility to be fibroids, tubal blockages, capacious uterine cavities, and hydrosalpinx.10–12 There is a dearth of similar data in Ghana. Therefore, we aimed to assess the patterns of HSG findings and the sociodemographic and clinical variables among Ghanaian women with infertility over a period of five years.
Methods
We undertook a retrospective review of all radiological reports of 2324 women with infertility who underwent HSG during their infertility work-up at the Department of Radiology, Cape Coast Teaching Hospital (CCTH), who met the inclusion criteria between January 2018 and December 2022. The CCTH is one of Ghana’s top medical research and referral institutions and receives patients referred from all over the country.
The Ethical Review Committee of CCTH cleared the study as per the 1975 Helsinki Declaration (Ref. CCTHERC/EC/2018/30). Although informed consent was not required for this retrospective study, patient anonymity and confidentiality were ensured.
The demographic and imaging data of all patients who underwent HSG during the selected period were retrieved from the hospital’s electronic patient database. The type of infertility of each patient was assessed using her gravidity, parity, and medical history. All the HSG reports were reviewed by three radiologists with over 10 years of experience in fluoroscopic examinations including HSG.
The inclusion criteria were cases of women who underwent HSG within the study period, and whose complete medical records including comprehensive HSG reports were available. The cases that did not meet these conditions were excluded.
All HSG procedures we reviewed were conducted as per our institutional protocol: HSG was performed during the patient’s proliferative phase (days six to 11) when there is no menstrual flow. A negative urine pregnancy test was required. The patient was administered a rectal diclofenac suppository (100 mg) and oral hyoscine butyl bromide tablets (60–80 mg), 30–50 minutes before the examination to help relieve pain and reduce the likelihood of tubal spasms. With the patient in the lithotomy position, a control image of the pelvis was taken. A vaginal speculum was inserted to assess the external cervical os, followed by cervical cannulation. About 10–30 mL of contrast medium (iopamidol) was administered under fluoroscopic guidance after expelling air bubbles. Once the uterine cavity was filled with the contrast medium, a radiographic image was taken using Shimadzu Flexavision (2012 model) digital fluoroscopy system (Shimadzu Corporation, Kyoto, Japan). HSG was reported to be normal when both tubes were visualized, normal in caliber and with free spillage of contrast medium into the peritoneum, with a normal outline of the uterine cavity and cervical canal. An abnormal HSG was reported when there was evidence of unilateral or bilateral tubal obstruction/dilatation, and/or uterine or cervical abnormality.
The uterine abnormalities (acquired or congenital) reviewed in this study comprised of irregular uterine outline, filling defects in the uterine cavity, Asherman’s syndrome, elongated uterine cavity, arcuate uterus, and bicornuate uterus. Cervical abnormalities included elongated cervical canal, irregular cervical outline, edematous cervix, and patulous cervix. Tubal abnormalities considered were beaded tube(s) indicative of tubercular salpingitis, salpingitis isthmica nodosa (bilateral or unilateral), hydrosalpinx(es) (bilateral or unilateral), terminal tubal contrast collection suggestive of fimbrial adhesion(s), and tubal blockage (bilateral or unilateral) indicated by the non-spillage of contrast medium to the affected side(s).
From the records, we classified all the patients into two infertility categories: primary and secondary. During the study period, a total of 2337 patients underwent HSG examination of whom 13 were excluded because of incomplete reports. The records of the remaining 2324 women who met the inclusion criteria were consecutively retrieved for analysis. The patients were categorized into the following age groups: ≤ 30 years, 31–35 years, 36–40 years, and > 40 years.
The collected data was analyzed and presented as tables and charts using GNU PSPP statistical analysis software version 1.2.0-3 (Free Software Foundation, Boston, Massachusetts, USA) and LibreOffice Calc version 6.1.5.2 (The Document Foundation, Berlin, Germany). The associations between the HSG findings and other variables—patient age, year of HSG, and type of infertility—were assessed using the chi-square goodness-of-fit test. After the assumption for normality check was satisfied, a two-tailed independent sampled students t-test was employed to determine whether the mean ages of patients with primary and secondary infertility were statistically the same in our setting. Statistical significance was set at p ≤ 0.05.
Results
The subjects of the study comprised of 2324 women with infertility who underwent HSG. The mean age of patients with primary infertility was 32.2±4.5 years while that of patients with secondary infertility was 34.2±5.3 years (p < 0.001). The percentage of the youngest women (≤ 30 years) undergoing HSG was the highest in 2022 (27.1%; p < 0.001) and the lowest in 2018 (13.2%; p < 0.001). A similar chronological trend was seen in the 31–35-year age group [Table 1]. The percentage of the oldest women (> 40 years) undergoing HSG was the highest in 2021 (33.9%) and the lowest in 2019 (6.6%). Women who presented with primary infertility constituted the majority of the patients with an accelerating yearly rate except in 2022. However, there was a fluctuating trend in the annual numbers of women with secondary infertility. These yearly changes were statistically significant (p < 0.001).
Table 1: Distribution of age, hysterosalpingographic (HSG) findings, and infertility types over a five-year period among Ghanaian women with infertility (N = 2324).
|
Age group, years
|
|
|
|
|
|
|
|
|
≤ 30
|
116 (13.2)
|
165 (18.8)
|
163 (18.5)
|
198 (22.5)
|
238 (27.0)
|
880 (100)
|
< 0.001*
|
|
31–35
|
122 (14.9)
|
131 (16.0)
|
158 (19.3)
|
192 (23.4)
|
216 (26.4)
|
819 (100)
|
|
|
36–40
|
78 (15.5)
|
72 (14.3)
|
72 (14.3)
|
90 (17.9)
|
192 (38.1)
|
504 (100)
|
|
|
> 40
|
21 (17.4)
|
8 (6.6)
|
33 (27.3)
|
41 (33.9)
|
18 (14.9)
|
121 (100)
|
|
|
Total
|
337 (14.5)
|
376 (16.2)
|
426 (18.3)
|
521 (22.4)
|
664 (28.6)
|
2324 (100)
|
|
|
Infertility type
|
|
Primary
|
266 (15.8)
|
308 (18.3)
|
326 (19.3)
|
440 (26.1)
|
345 (20.5)
|
1685 (100)
|
< 0.001*
|
|
Secondary
|
71 (11.1)
|
68 (10.6)
|
100 (15.6)
|
81 (12.7)
|
319 (49.9)
|
639 (100)
|
|
|
Overall HSG findings
|
|
Normal
|
72 (14.0)
|
60 (11.7)
|
72 (14.0)
|
135 (26.3)
|
174 (33.9)
|
513 (100)
|
< 0.001*
|
|
Abnormal
|
265 (14.6)
|
316 (17.4)
|
354 (19.5)
|
386 (21.3)
|
490 (27.1)
|
1811 (100)
|
|
|
Age, years
|
21
|
48
|
32.7 (4.8)
|
0.100
|
|
Primary
|
32.2 (4.5)
|
-1.99 (-2.42–1.56)
|
-9.02
|
< 0.001*
|
*Significance. Diff: difference. N: total number of women who underwent HSG.
HSG was unable to identify any signs of infertility in respect of 513 (22.1%) participants despite their known clinical infertility [Table 1]. Thus, the net number of women with fertility abnormalities as found by HSG was 1811.
Primary infertility cases exceeded secondary infertility cases in all age groups with an inverse relationship with age [Figure 1].

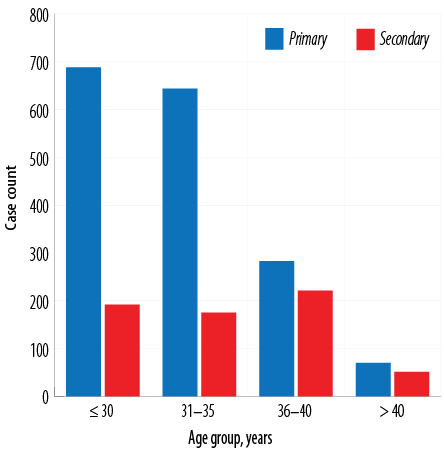 Figure 1: Prevalence of primary and secondary infertility in Ghanaian women stratified by age group.
Figure 1: Prevalence of primary and secondary infertility in Ghanaian women stratified by age group.
The most common abnormal HSG finding was bilateral tubal blockage (58.9%), followed by elongated cervical canal (25.1%) and irregular uterine outline (1.4%). The least recorded findings were Asherman’s syndrome, bilateral beaded tubes/tubercular salpingitis, and bicornuate uterus. Apart from left hydrosalpinx, the positive HSG findings were more prevalent in primary infertility cases. All the cases of right tubal blockage, right fimbrial adhesions, edematous cervix, patulous cervix [Figure 2], arcuate uterus, bicornuate uterus, Asherman’s syndrome, and bilateral beaded tubes/tubercular salpingitis were found in women with primary infertility [Table 2]. Hydrosalpinx was recorded in 72.9% of primary and 27.1% of secondary infertility cases and was predominantly on the left side. Fimbrial adhesions were seen in 21.5% of primary and 12.8% of secondary infertility cases and were more common on the right side [Table 2].

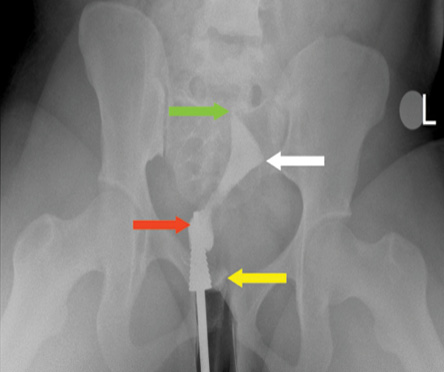 Figure 2: A frontal hysterosalpingography image showing pooling of contrast medium around the cervical cannula suggestive of a patulous cervix as indicated by the red arrow, backflow of contrast (yellow arrow), right tubal blockage (green arrow), and left tubal blockage (white arrow).
Figure 2: A frontal hysterosalpingography image showing pooling of contrast medium around the cervical cannula suggestive of a patulous cervix as indicated by the red arrow, backflow of contrast (yellow arrow), right tubal blockage (green arrow), and left tubal blockage (white arrow).
Table 2: Abnormal hysterosalpingographic (HSG) findings in Ghanaian women with primary versus secondary infertility (n = 1811).
|
Tubal findings
|
|
Bilateral tubal blockage
|
701 (65.8)
|
365 (34.2)
|
1066
|
< 0.001*
|
|
Right tubal blockage
|
275 (100)
|
0 (0.0)
|
275
|
< 0.001*
|
|
Left tubal blockage
|
157 (97.5)
|
4 (2.5)
|
161
|
< 0.001*
|
|
Bilateral hydrosalpinxes
|
78 (72.9)
|
29 (27.1)
|
107
|
0.926
|
|
Left hydrosalpinx
|
51 (39.53)
|
78 (60.5)
|
129
|
< 0.001*
|
|
Right fimbrial adhesions
|
172 (100)
|
0 (0.0)
|
172
|
< 0.001*
|
|
Left fimbrial adhesions
|
190 (69.9)
|
82 (30.1)
|
272
|
0.297
|
|
Bilateral beaded tubes/
tubercular salpingitis
|
5 (100)
|
0 (0.0)
|
5
|
0.168
|
|
Bilateral tubal patency
|
446 (63.5)
|
256 (36.5)
|
702
|
< 0.001*
|
|
Uterine findings
|
|
Elongated uterine cavity
|
39 (76.5)
|
12 (23.5)
|
51
|
0.521
|
|
Irregular uterine outline
|
229 (78.7)
|
62 (21.3)
|
291
|
0.011*
|
|
Filling defect/fibroid
|
98 (61.3)
|
62 (38.8)
|
160
|
0.001*
|
|
Arcuate uterus
|
16 (100)
|
0 (0.0)
|
16
|
0.013*
|
|
Bicornuate uterus
|
7 (100)
|
0 (0.0)
|
7
|
0.103
|
|
Asherman’s syndrome
|
4 (100)
|
0 (0.0)
|
4
|
0.218
|
|
Cervical findings
|
|
Elongated cervical canal
|
298 (65.6)
|
156 (34.4)
|
454
|
< 0.001*
|
|
Irregular cervical outline
|
22 (84.6)
|
4 (15.4)
|
26
|
0.164
|
|
Edematous cervix
|
135 (100)
|
0 (0.0)
|
135
|
< 0.001*
|
*Significance. n: total number of patients with abnormal HSG findings.
Irregular uterine outline, bilateral tubal blockage [Figure 2], left tubal blockage, elongated cervical canal, elongated uterine cavity, irregular cervical outline, and edematous cervix were significantly associated with the youngest (≤ 30 years) age group. Filling defects/fibroids, bicornuate uterus, and bilateral beaded tubes/tubercular salpingitis were more prevalent among 36–40-year age group. Right tubal blockage, bilateral hydrosalpinxes, [Figure 3], left hydrosalpinx, right fimbrial adhesions, left fimbrial adhesions, patulous cervix, arcuate uterus, and Asherman’s syndrome were more likely among 31–35-year-olds [Table 3].

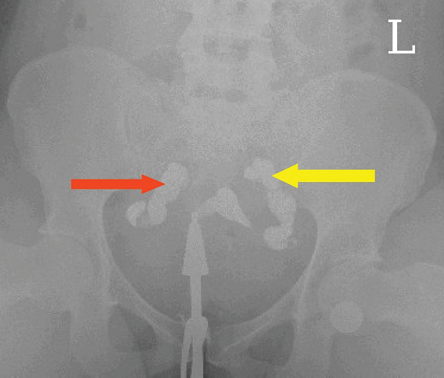 Figure 3: A hysterosalpingography image showing bilateral tubal dilatation with no spillage of contrast medium bilaterally into the peritoneal cavity as depicted by the left and right arrows suggestive of bilateral hydrosalpinxes.
Figure 3: A hysterosalpingography image showing bilateral tubal dilatation with no spillage of contrast medium bilaterally into the peritoneal cavity as depicted by the left and right arrows suggestive of bilateral hydrosalpinxes.
Table 3: Prevalence of abnormal hysterosalpingographic (HSG) findings in various age groups (n = 1811).
|
Tubal findings
|
|
Bilateral tubal blockage
|
428 (40.2)
|
341 (32.0)
|
232 (21.8)
|
65 (6.10)
|
1066
|
0.009*
|
|
Right tubal blockage
|
98 (35.6)
|
138 (50.2)
|
39 (14.2)
|
0 (0.0)
|
275
|
< 0.001*
|
|
Left tubal blockage
|
56 (34.8)
|
38 (23.6)
|
47 (29.2)
|
20 (12.4)
|
161
|
< 0.001*
|
|
Bilateral hydrosalpinxes
|
23 (21.5)
|
38 (35.5)
|
14 (13.1)
|
32 (29.9)
|
107
|
< 0.001*
|
|
Left hydrosalpinx
|
15 (11.6)
|
53 (41.1)
|
50 (38.8)
|
11 (8.5)
|
129
|
< 0.001*
|
|
Right fimbrial adhesions
|
69 (40.1)
|
92 (53.5)
|
4 (2.3)
|
7 (4.1)
|
172
|
< 0.001*
|
|
Left fimbrial adhesions
|
72 (26.5)
|
112 (41.2)
|
84 (30.9)
|
4 (1.5)
|
272
|
< 0.001*
|
|
Bilateral beaded tubes/
tubercular salpingitis
|
0 (0.0)
|
0 (0.0)
|
5 (100)
|
0 (0.0)
|
5
|
< 0.001*
|
|
Bilateral tubal patency
|
264 (37.6)
|
250 (35.6)
|
174 (24.8)
|
14 (2.0)
|
702
|
< 0.001*
|
|
Uterine findings
|
|
Elongated uterine cavity
|
33 (64.7)
|
18 (35.3)
|
0 (0.0)
|
0 (0.0)
|
51
|
< 0.001*
|
|
Irregular uterine outline
|
118 (40.5)
|
68 (23.37)
|
95 (32.6)
|
10 (3.4)
|
291
|
< 0.001*
|
|
Filling defect/fibroid
|
42 (26.3)
|
32 (20.0)
|
76 (47.5)
|
10 (6.3)
|
160
|
< 0.001*
|
|
Arcuate uterus
|
0 (0.0)
|
12 (75.0)
|
4 (25.0)
|
0 (0.0)
|
16
|
0.003*
|
|
Bicornuate uterus
|
0 (0.0)
|
0 (0.0)
|
7 (100)
|
0 (0.0)
|
7
|
< 0.001*
|
|
Asherman’s syndrome
|
1 (25.0)
|
3 (75.0)
|
0 (0.0)
|
0 (0.0)
|
4
|
0.384
|
|
Cervical findings
|
|
Elongated cervical canal
|
163 (35.9)
|
121 (26.7)
|
149 (32.8)
|
21 (4.6)
|
454
|
< 0.001*
|
|
Irregular cervical outline
|
14 (53.8)
|
7 (26.9)
|
5 (19.2)
|
0 (0.0)
|
26
|
0.292
|
|
Edematous cervix
|
76 (56.3)
|
40 (29.6)
|
19 (14.1)
|
0 (0.0)
|
135
|
< 0.001*
|
*Significance. n: total number of patients with abnormal HSG findings.

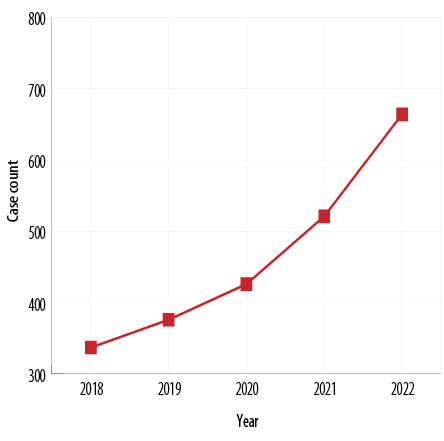 Figure 4: Yearly trend of infertility from 2018–2022 among Ghanaian women.
Figure 4: Yearly trend of infertility from 2018–2022 among Ghanaian women.

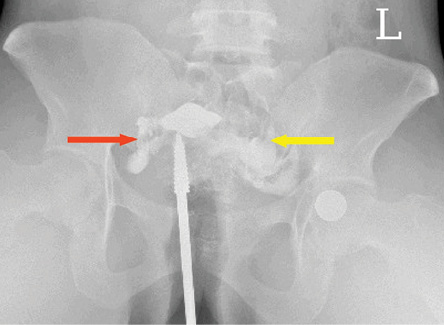 Figure 5: A frontal hysterosalpingography radiograph showing free intraperitoneal bilateral spillage (right spillage: red arrow and left spillage: yellow arrow) suggestive of bilateral tubal patency.
Figure 5: A frontal hysterosalpingography radiograph showing free intraperitoneal bilateral spillage (right spillage: red arrow and left spillage: yellow arrow) suggestive of bilateral tubal patency.
Table 4: Annual trend of the hysterosalpingographic (HSG) findings among Ghanaian women with infertility (N = 2324).
|
Tubal findings
|
|
|
|
|
|
|
|
|
Bilateral tubal blockage
|
158 (14.8)
|
188 (17.6)
|
220 (20.6)
|
252 (23.6)
|
248 (23.3)
|
1066
|
< 0.001*
|
|
Right tubal blockage
|
21 (7.6)
|
48 (17.5)
|
34 (12.4)
|
51 (18.5)
|
121 (44.0)
|
275
|
< 0.001*
|
|
Left tubal blockage
|
22 (13.7)
|
8 (5.0)
|
13 (8.1)
|
31 (19.3)
|
87 (54.0)
|
161
|
< 0.001*
|
|
Bilateral hydrosalpinxes
|
7 (6.5)
|
20 (18.7)
|
33 (30.8)
|
30 (28.0)
|
17 (15.9)
|
107
|
< 0.001*
|
|
Left hydrosalpinx
|
28 (21.7)
|
28 (21.7)
|
36 (27.9)
|
20 (15.5)
|
17 (13.2)
|
127
|
< 0.001*
|
|
Right fimbrial adhesions
|
43 (25.0)
|
32 (18.6)
|
43 (25.0)
|
20 (11.6)
|
34 (19.8)
|
172
|
< 0.001*
|
|
Left fimbrial adhesions
|
35 (12.9)
|
72 (26.5)
|
52 (19.1)
|
45 (16.5)
|
68 (25.0)
|
272
|
< 0.001*
|
|
Beaded tubes/tubercular salpingitis
|
0 (0.0)
|
0 (0.0)
|
0 (0.0)
|
5 (100)
|
0 (0.0)
|
5
|
0.002*
|
|
Bilateral tubal patency
|
107 (15.2)
|
108 (15.4)
|
124 (17.7)
|
172 (24.5)
|
191 (27.2)
|
702
|
0.460
|
|
Uterine findings
|
|
|
|
|
|
|
|
|
Elongated uterine cavity
|
8 (15.7)
|
12 (23.5)
|
9 (17.6)
|
5 (9.8)
|
17 (33.3)
|
51
|
0.205
|
|
Irregular uterine outline
|
35 (12.0)
|
80 (27.5)
|
71 (24.4)
|
87 (29.9)
|
18 (6.2)
|
291
|
< 0.001*
|
|
Filling defect/fibroid
|
28 (17.5)
|
36 (22.5)
|
41 (25.6)
|
37 (23.1)
|
18 (11.3)
|
160
|
< 0.001*
|
|
Arcuate uterus
|
8 (50.0)
|
0 (0.0)
|
8 (50.0)
|
0 (0.0)
|
0 (0.0)
|
16
|
< 0.001*
|
|
Bicornuate uterus
|
7 (100.0)
|
0 (0.0)
|
0 (0.0)
|
0 (0.0)
|
0 (0.0)
|
7
|
< 0.001*
|
|
Asherman’s syndrome
|
0 (0.0)
|
4 (100)
|
0 (0.0)
|
0 (0.0)
|
0 (0.0)
|
4
|
< 0.001*
|
|
Cervical findings
|
|
|
|
|
|
|
|
|
Elongated cervical canal
|
64 (14.1)
|
120 (26.4)
|
111 (24.4)
|
107 (23.6)
|
52 (11.5)
|
454
|
< 0.001*
|
|
Irregular cervical outline
|
7 (26.92)
|
4 (15.38)
|
5 (19.23)
|
10 (38.46)
|
0 (0.0)
|
26
|
0.010*
|
|
Edematous cervix
|
7 (5.2)
|
44 (32.6)
|
34 (25.2)
|
50 (37.0)
|
0 (0.0)
|
135
|
< 0.001*
|
*Significance. N: total number of participants.
Figure 4 shows an increasing trend of infertility among Ghanaian women over five years at an accelerating rate.
The year 2021 saw the highest rates of irregular uterine outline (29.9%), bilateral tubal blockage (23.6%), irregular cervical outline (38.5%), edematous cervix (37.0%), and bilateral beaded tubes/tubercular salpingitis. Meanwhile, right tubal blockage (44.0%), left tubal blockage (54.0%), elongated uterine cavity (33.3%), and bilateral tubal patency (27.2%) [Figure 5] were the most common in 2022. All findings of bicornuate uterus, Asherman’s syndrome, and bilateral beaded tubes/tubercular salpingitis features occurred in 2018, 2019, and 2021, respectively [Table 4].
Discussion
According to the literature, secondary infertility is the most prevalent type of female infertility worldwide.13 However, a large majority (72.5%) of the women in this study had primary infertility, mainly resulting from bilateral tubal blockage (41.6%). Bilateral tubal blockage also accounted for 57.1% of secondary infertility and 45.9% of all infertility cases. Ambildhuke et al,14 reported bilateral tubal obstruction as responsible for up to 40% of female infertility which supports our finding. In another study, bilateral tubal blockage constituted the majority, however, unlike our findings, only 22.0% of their participants had primary infertility.15
A Nigerian study reported that nearly half (44.8%) of their patients had primary infertility, which was higher than the international prevalence and thus closer to ours.16 We found that primary infertility was more common in younger women (≤ 30 years) and had a decreasing trend with age. On the other hand, secondary infertility was more prevalent in the 36–40 years age group, with a fluctuating pattern with increasing age [Figure 1]. The Nigerian study also reported a high prevalence of primary infertility among younger women (< 30 years) with a similar pattern as ours, while secondary infertility was common among those aged 35–39 years.16 A study from Sudan found a similar pattern for primary infertility, but the secondary infertility increased with increasing age against our fluctuating trend.17 A large long-term study in the UK reported that the causes of infertility in older women differed from those in younger women.18
Our participants with primary infertility were significantly younger than those with secondary infertility [Table 1], which was corroborated by other studies.17,19 All our patients with right tubal blockage, right fimbrial adhesions, and edematous cervix among others had primary infertility
[Table 2] and only a small minority (0.17–0.69%) had these abnormal findings, similar to findings in other studies.10,20
Past research suggested that myomas, intrauterine adhesions, polyps, and gas bubbles (iatrogenic) may show up as filling defects on HSG. Filling defects were recorded in 6.9% of all our patients. A study in Nigeria reported a much higher proportion of filling defects (26.9%).21
The outline of the uterus was irregular in 13.4% of our patients and the cervical outline was irregular in 1.6%, against 20.6% and 12.1%, respectively in the Nigerian study.21 Uterine abnormalities were seen in 22.8% of our patients, attributable to genetic, acquired, and environmental factors.22 Genes such as Pax, Wnt9b, Wnt4, Emx2, and Lim1 have been reported to influence the development of Mullerian ducts. Among environmental triggers is exposure to diethylstilbestrol and thalidomide during pregnancy, which may cause malformations such as T-shaped uterus in the fetus.22,23 A study in Oman found a much higher prevalence of uterine abnormalities than among our participants (3% versus 1%).24 The possible reason for their higher proportion is due to the inclusion of only congenital uterine abnormalities.
Cervical abnormalities were observed in 27.0% of all our patients against 10% reported in the literature.25 The comparatively high prevalence of cervical abnormalities in women with infertility could be caused by uterine prolapse and irregular and edematous cervix due to chronic cervicitis. Studies have associated uterine prolapse with elongation of the supravaginal part of the cervix while the vaginal part has been linked with chronic cervicitis leading to cervical hypertrophy.26,27
Asherman’s syndrome was found in 0.2% of our patients against a much higher prevalence reported in the literature. A systematic review conducted in Denmark reported a prevalence range of 2.8–45.5%, while 1.5% was reported in the USA.28,29 The extremely low prevalence of Asherman’s syndrome in our study is positive for Ghanaian women, due to its association with menstrual disturbances, recurrent pregnancy losses, and infertility.28,30 Asherman’s syndrome is generally associated with secondary infertility,30,31 while the opposite was recorded in our study.
Hydrosalpinx was found in 10.2% of our participants. A European survey recorded a 30% prevalence. The majority of our cases of hydrosalpinx were on the left side with decreasing patterns as the years progressed [Table 4]. However, a Nigerian study reported a higher incidence of hydrosalpinx on the right side, probably due to the presence of the appendix.32
In addition, 19.1% of our patients had fimbrial adhesions and 0.2% had bilateral beaded tubes/tubercular salpingitis. About 22.1% of the infertility cases in this study were not identifiable on HSG, possibly due to factors beyond the scope of HSG such as ovulation problems, poor sperm, or egg quality, etc.2
Generally, we observed an increasing trend in the annual cases of infertility at an accelerating rate as the years progressed [Figure 2]. There was no clear pattern seen in the annual proportions of HSG findings apart from tubal blockage which appeared to be increasing with progressing years. Our finding has been corroborated by Sun et al,33’s massive long-term study covering 195 countries and territories worldwide, which found that the international prevalence rate of infertility has been growing by 0.29% for men and 0.37% for women.
Our study has limitations. Firstly, the causal relationship between primary and secondary infertility could not be evaluated in this study. Secondly, the sample size was slightly reduced due to the exclusion of patients whose files lacked comprehensive medical records and HSG reports. Finally, the largely homogenous characteristics of our study population may impact the generalization of our findings.
As 22.1% of infertility cases could not be identified on HSG, practitioners should explore other causal factors such as ovulation problems and poor sperm/egg quality. The rising trend in the annual number of patients undergoing HSG is an evolving public health concern. This calls for policy measures to manage this serious long-term problem, along with its associated medical, economic, and psychosocial fallouts, particularly in Africa where there is a strong emphasis on childbearing.
Conclusion
Most Ghanaian women in this study had primary infertility with a progressively accelerating rate over the study period. The prevalence of secondary infertility fluctuated during the same period. Women with primary infertility were significantly younger than those with secondary infertility. Tubal blockage and cervical abnormalities were the most prevalent HSG findings while Asherman’s syndrome and bilateral beaded tubes/tubercular salpingitis were the least prevalent. There was no clear pattern seen in the annual proportions of HSG findings apart from tubal blockage which appeared to be increasing with progressing years. These increases are part of the global rise in female infertility, in addition to the associated socio-psychological sequelae.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgments
The management of Cape Coast Teaching Hospital and the workers of the radiology unit are appreciated for their support in making this study successful.
references
- 1. Sormunen T, Aanesen A, Fossum B, Karlgren K, Westerbotn M. Infertility-related communication and coping strategies among women affected by primary or secondary infertility. J Clin Nurs 2018 Jan;27(1-2):e335-e344.
- 2. Deshpande PS, Gupta AS. Causes and prevalence of factors causing infertility in a public health facility. J Hum Reprod Sci 2019;12(4):287-293.
- 3. World Health Organization. WHO fact sheet on infertility. Glob Reprod Health 2021 Apr;6(1):e52.
- 4. Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci 2015;8(4):191-196.
- 5. Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982-2010: data from the national survey of family growth. National Health Statistics Reports. 2013 [cited 2023 July 4]. Available from: https://www.cdc.gov/nchs/data/nhsr/nhsr067.pdf.
- 6. Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 2012;9(12):e1001356.
- 7. Bhardwaj JK, Panchal H, Saraf P. Ameliorating effects of natural antioxidant compounds on female infertility: a review. Reprod Sci 2021 May;28(5):1227-1256.
- 8. Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril 2012 Aug;98(2):302-307.
- 9. Preutthipan S, Linasmita V. A prospective comparative study between hysterosalpingography and hysteroscopy in the detection of intrauterine pathology in patients with infertility. J Obstet Gynaecol Res 2003 Feb;29(1):33-37.
- 10. Makwe CC, Ugwu AO, Sunmonu OH, Yusuf-Awesu SA, Ani-Ugwu NK, Olumakinwa OE. Hysterosalpingography findings of female partners of infertile couple attending fertility clinic at Lagos University Teaching Hospital. Pan Afr Med J 2021 Dec;40(1):223.
- 11. Bukar M, Mustapha Z, Takai UI, Tahir A. Hysterosalpingographic findings in infertile women: a seven year review. Niger. J. Clin. Pract 2011;14(2):168-170.
- 12. Kiguli-Malwadde E, Byanyima RK. Structural findings at hysterosalpingography in patients with infertility at two private clinics in Kampala, Uganda. Afr Health Sci 2004 Dec;4(3):178-181.
- 13. Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem 2018 Dec;62:2-10.
- 14. Ambildhuke K, Pajai S, Chimegave A, Mundhada R, Kabra P. A Review of tubal factors affecting fertility and its management. Cureus 2022 Nov;14(11):e30990.
- 15. Kiridi EK, Oriji PC, Ugwoegbu JU, Abasi IJ. Hysterosalpingography findings among women presenting for infertility evaluation in Bayelsa state, South-South Nigeria. J Adv Med Med Res 2022 Mar;34(5):7-17.
- 16. Okafor CO, Okafor CI, Okpala OC, Umeh E. The pattern of hysterosalpingographic findings in women being investigated for infertility in Nnewi, Nigeria. Niger J Clin Pract 2010 Sep;13(3):264-267.
- 17. Toufig H, Benameur T, Twfieg ME, Omer H, El-Musharaf T. Evaluation of hysterosalpingographic findings among patients presenting with infertility. Saudi J Biol Sci 2020 Nov;27(11):2876-2882.
- 18. Maheshwari A, Hamilton M, Bhattacharya S. Effect of female age on the diagnostic categories of infertility. Hum Reprod 2008 Mar;23(3):538-542.
- 19. Benksim A, Elkhoudri N, Addi RA, Baali A, Cherkaoui M. Difference between primary and secondary infertility in Morocco: frequencies and associated factors. Int J Fertil Steril 2018 Jul;12(2):142-146.
- 20. Botwe BO, Bamfo-Quaicoe K, Hunu E, Anim-Sampong S. Hysterosalpingographic findings among Ghanaian women undergoing infertility work-up: a study at the Korle-Bu Teaching Hospital. Fertil Res Pract 2015 Jun;1:9.
- 21. Udobi SI, Aronu ME. Hysterosalpingographic findings in women with infertility in Awka, Anambra State, South-East Nigeria. Niger Surg Sci 2017 Jul;27(2):47-50.
- 22. Kaur P, Panneerselvam D. Bicornuate uterus. StatPearls 2023 [cited 2023 July 1]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560859/.
- 23. Roly ZY, Backhouse B, Cutting A, Tan TY, Sinclair AH, Ayers KL, et al. The cell biology and molecular genetics of Müllerian duct development. Wiley Interdiscip Rev Dev Biol 2018 May;7(3):e310.
- 24. Albalushi H, Ba-Alawi A, Aljabri R, Al Khaduri M. Prevalence of congenital uterine anomalies and tubal blockage in infertile Omani women: a retrospective study. Oman Med J 2023 Jan;38(1):e463.
- 25. Zafarani F, Ahmadi F, Shahrzad G. Hysterosalpingographic features of cervical abnormalities: acquired structural anomalies. Br J Radiol 2015 Aug;88(1052):20150045.
- 26. Hiremath PB, Bansal N, Hiremath R. Extreme cervical elongation. Int J Reprod Contracept Obstet Gynecol 2014 Sep;3(3):777-780.
- 27. Shemer O, Vinikov Y, Shaubi-Rosen M, Levy G. Cervical elongation - the search for a definition. Maedica (Bucur) 2022 Jun;17(2):487-491.
- 28. Dreisler E, Kjer JJ. Asherman’s syndrome: current perspectives on diagnosis and management. Int J Womens Health 2019 Mar;11:191-198.
- 29. Smikle C, Yarrarapu SN, Khetarpal S. Asherman syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2023 July 1]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448088/.
- 30. Cenksoy PO, Ficicioglu C, Yesiladali M, Kizilkale O. The diagnosis and management of Asherman’s syndrome developed after cesarean section and reproductive outcome. Case Rep Obstet Gynecol 2013;2013:450658.
- 31. Tsui KH, Lin LT, Cheng JT, Teng SW, Wang PH. Comprehensive treatment for infertile women with severe Asherman syndrome. Taiwan J Obstet Gynecol 2014 Sep;53(3):372-375.
- 32. Adedigba JA, Idowu BM, Hermans SP, Ibitoye BO, Fawole OA. The relationship between hysterosalpingography findings and female infertility in a Nigerian population. Pol J Radiol 2020 Apr;85(1):e188-e195.
- 33. Sun H, Gong TT, Jiang YT, Zhang S, Zhao YH, Wu QJ. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: results from a global burden of disease study, 2017. Aging (Albany NY) 2019 Dec;11(23):10952-10991.