Ameloblastoma is an odontogenic tumors that comprises about 11% of odontogenic tumors and only 1% of all solid tumors involving the oral cavity.1 The World Health Organization (WHO) defines ameloblastoma as a locally invasive and histologically heterogeneous tumor with high recurrence rate.2 Ameloblastoma poses a challenging clinical course in almost every patient due to the lack of reliable clinical and pathological factors predicting its biological behavior.3 The use of cell proliferation biomarkers (e.g., Ki-67 and proliferating cell nuclear antigen) and tumor suppressor genes (e.g., p53 and breast cancer susceptibility gene 1) has been reported for predicting the biological behavior of numerous tumors including ameloblastoma.1–4 For instance, Migaldi et al,5 reported that increased expression of Ki-67 was associated with increased microsatellite instabilities, a high rate of recurrence, and short disease-free survival among patients with ameloblastoma. The quantity and quality of p53 expression involve a more aggressive behavior of cancers, and therefore, its overexpression can promote cell proliferation in odontogenic lesions.6
Ki-67 is a non-histone nucleoprotein in cells, which increases its proliferation as cells prepare to divide into new cells.7 Two isoforms of Ki-67 antigen, namely 345 and 395 kDa, have been identified.8 Ki-67-positive nuclei in the ameloblastoma are mainly located in peripheral ameloblast-like cells in follicular as well as plexiform variants of the conventional type and in the basal cells of unicystic ameloblastoma.9 Stellate reticulum-like cells in ameloblastoma and those in developing teeth tend to be negative for this biomarker. This staining pattern indicates that the cellular proliferation and consequently the growth of ameloblastoma are concentrated in the peripheral areas composed of ameloblast-like cells.10 One study found that Ki-67 expression was higher in ameloblastic carcinoma compared to benign ameloblastoma, suggesting that this biomarker can be useful when considering the diagnosis of malignancy and perhaps could play a role in malignant transformation of ameloblastoma.11
p53 is a tumor suppressor gene located on chromosome 17p13.1, which codes for a protein that regulates the cell cycle and hence plays a role in tumor suppression.12 One review article reported that the expression of p53 in the plexiform pattern was 25% and in the follicular pattern was 20%.13 Hirayama et al,14 reported expression of p53 of 43% for the plexiform pattern and 37% for the follicular pattern. One study reported that p53 expression was higher in ameloblastic carcinoma compared to benign ameloblastoma, suggesting that this biomarker can be useful when considering the diagnosis of malignancy and perhaps could play a role in malignant transformation of ameloblastoma. Apart from unicystic histological subtype with well-established better prognosis by virtual of not being locally very aggressive,3 other clinical and pathological factors have been reported to
be controversial.1
There is a gap in knowledge and practice regarding the establishment of serum biomarkers and/or molecular prognostic factors that can reliably predict the biological behavior of ameloblastoma. Therefore, this study aimed to determine the immunohistochemical (IHC) expression of Ki-67 and p53 and the factors associated with their expression among patients with ameloblastoma.
Methods
This was a retrospective longitudinal study conducted at Makerere College of Health Sciences in Kampala, Uganda. Retrospective data of patients who were confirmed histologically with ameloblastoma, treated surgically, and followed-up for seven years (January 2012 to December 2018) were extracted for analysis.
Patients with a confirmed histological diagnosis of ameloblastoma, complete clinical and follow-up data, and available formalin-fixed paraffin-embedded tissue block were analyzed. However, patients with missing or spoiled tissue blocks and those with incomplete or missing clinical and follow-up data were excluded from the analysis.
The sample size of 40 cases was obtained conveniently, and all available cases meeting the inclusion criteria were recruited simultaneously and included in the study.
Recurrence was defined as confirmation of the disease either clinically or radiologically after surgical removal of the primary tumor. This work has been reported in line with the strengthening the reporting of cohort, cross-sectional and case-control studies in surgery criteria.15 Histological classification of the subtypes of ameloblastoma was based on the current 2017 WHO classification system.16
Re-confirmation of the previous histological diagnosis using routine hematoxylin and eosin stains was done. Then, we classified histologically the cases based on the new WHO classification of ameloblastoma of 2017.17 IHC staining for monoclonal mouse antibodies (Dako) was used to evaluate the expression of the proteins of interest (p53 – clone DO-7 and Ki-67 – clone MIB-1) by adapting the protocol from a previous study.6 The serial sections were cut at the thickness of 4 microns and then were de-waxed by heating them on a hot plate at 60 oC for 30 min, followed by clearing in three changes of xylene. The sections were brought down to water by dipping them in descending concentrations of alcohol and then rinsed in distilled water. Two drops of 3% H2O2 solution were added to each section for 15 min to block endogenous peroxidase, and then, the slides were rinsed in distilled water. The slides were incubated in the antigen retrieval solution citrate buffer of pH 6.0 within a pressure cooker at 95 °C, from which the slides were removed after 2 min of full pressure.
Heat-induced epitope retrieval method in microwave oven for 30 min in Tris-EDTA buffer solution pH 9.0 for p53 protein and in citrate buffer solution pH 6.1 for Ki-67 antigen. Tris-EDTA buffer solution was then drained from slides and a ring was made around the section using Pap pen (Sigma-Aldrich Labware, German) to limit spreading of antibody solutions. The sections were then incubated with MIB-1 monoclonal antibody (mouse monoclonal antibody, dilution of 1:200, Dako) and anti-p53 monoclonal antibodies (mouse clone DO-7, dilution 1:50, Dako) and for 3 min at room temperature simultaneously. Then, 2,3-diaminobenzidine was used as a chromogenic substrate and Dako LSAB2 as a detection system. The sections were washed in distilled water, counterstained in hematoxylin for 10 sec, and differentiated by 2 dips into 1% acid alcohol. The sections were blued in warm water for 2 min, dehydrated through 70%, 80%, 95%, and 100% ethanol, and then cleared in xylene for 10 min. The sections were finally cover-slipped using distyrene plasticizer xylene and were ready for scoring. Positive staining was defined by a presence of brownish intranuclear staining in tumor cells for both Ki-67 and p53. For validation of the IHC results, a known case of breast cancer was used as a positive control for both Ki-67 and p53 proteins, and removal of the primary antibodies was taken as negative control for Ki-67 and p53 expression.18
The labeling index (LI) for both Ki-67 and p53 was calculated as the number of positive cells × 100/total number of cells (positive + negative) at high magnification (400 ×) as previously.19 Counting of the positive tumor cells was done manually from different compartments (basal, granular, squamous, stellate, and reticulum). Ki-67 LI of ≤ 20% was considered low and > 20% was regarded to be high, as done in a previous study.20 The p53 LI was regarded high if the expression was ≥ 10% and low if < 10% as previous.21 This was followed by determining the staining intensity for both antibodies, and the intensity was labeled as 1, 2, and 3 for weak, moderate, and strong intranuclear staining, respectively.
Analysis of the data was done using SPSS Statistics (IBM IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.). Assessment of factors associated with Ki-67 and p53 expression was done using one-way analysis of variance, and for variables with more than three groups, post hoc test was performed to determine which group was different from other groups. Factors associated with recurrence were assessed using chi-square and Fisher’s exact tests where appropriate. The level of statistical significance was set at a 95% CI with p < 0.05.
Results
The sociodemographic and clinical characteristics of the patients and the expression of Ki-67 and p53 are presented in Table 1. A total of 40 patients who were confirmed histologically with ameloblastoma were analyzed. The mean age of the patients was 33.6±14.4 years with an age range of 9–67 years. Males were slightly higher (n = 23; 57.5%) than females with a male to female ratio of 1.4:1. The mean duration from onset of symptoms to hospitalization was 9.2±6.9 months (range = 2–30 months). Most (n = 23, 57.5%) of the patients sought medical services beyond six months since the onset of the symptoms. The mean tumor size was 4.8±2.2 cm and the majority (n = 28; 70.0%) of patients had tumor size greater than the mean tumor size. Over half (n = 22; 55.0%) of the tumors were located in the posterior part of the mandible.
Table 1: Clinical characteristics and Ki-67 and p53 expression (N = 40).
|
1
|
38
|
F
|
3
|
3.0
|
MA
|
U
|
N
|
38.0
|
CT
|
0.0
|
0
|
3.2
|
3
|
|
2
|
21
|
M
|
4
|
3.2
|
MP
|
C
|
N
|
30.0
|
CT
|
10.6
|
1
|
0.0
|
0
|
|
3
|
34
|
M
|
2
|
4.2
|
MXP
|
U
|
N
|
49.0
|
CT
|
8.0
|
1
|
2.3
|
3
|
|
4
|
32
|
M
|
5
|
2.3
|
MP
|
U
|
N
|
100.0
|
CT
|
3.5
|
1
|
0.0
|
0
|
|
5
|
41
|
F
|
8
|
8.4
|
MP
|
C
|
Y
|
60.0
|
SR
|
22.2
|
3
|
79.5
|
3
|
|
6
|
48
|
F
|
2
|
3.1
|
MP
|
U
|
N
|
56.0
|
CT
|
0.0
|
0
|
3.4
|
3
|
|
7
|
20
|
F
|
4
|
3.4
|
MP
|
C
|
N
|
40.0
|
CT
|
3.6
|
2
|
2.3
|
3
|
|
8
|
18
|
M
|
5
|
3.5
|
MA
|
U
|
N
|
35.0
|
CT
|
2.8
|
3
|
3.6
|
3
|
|
9
|
62
|
F
|
3
|
2.4
|
MP
|
C
|
N
|
102.0
|
CT
|
15.8
|
3
|
3.4
|
3
|
|
10
|
29
|
M
|
2
|
4.1
|
MP
|
C
|
N
|
80.0
|
CT
|
3.6
|
3
|
24.7
|
3
|
|
11
|
56
|
M
|
6
|
6.8
|
MA
|
C
|
Y
|
48.0
|
SR
|
34.8
|
1
|
12.5
|
0
|
|
12
|
40
|
F
|
7
|
7.1
|
MP
|
C
|
Y
|
48.0
|
SR
|
25.9
|
1
|
0.0
|
0
|
|
13
|
19
|
M
|
3
|
3.4
|
MP
|
U
|
N
|
50.0
|
CT
|
0.0
|
0
|
33.2
|
3
|
|
14
|
9
|
M
|
5
|
3.5
|
MP
|
C
|
N
|
45.0
|
CT
|
0.0
|
0
|
0.0
|
0
|
|
15
|
53
|
M
|
8
|
4.3
|
MXP
|
U
|
Y
|
12.0
|
SR
|
8.15
|
3
|
1.7
|
1
|
|
16
|
14
|
M
|
2
|
4.2
|
MXP
|
U
|
N
|
20.0
|
CT
|
17.9
|
3
|
76.5
|
3
|
|
17
|
21
|
F
|
15
|
6.2
|
MP
|
U
|
Y
|
84.0
|
CT
|
20.8
|
1
|
18.5
|
3
|
|
18
|
26
|
M
|
7
|
2.5
|
MA
|
U
|
N
|
45.0
|
CT
|
3.2
|
3
|
9.4
|
3
|
|
19
|
21
|
F
|
24
|
5.1
|
MP
|
C
|
Y
|
28.0
|
SR
|
9.55
|
3
|
35.4
|
0
|
|
20
|
21
|
M
|
9
|
3.4
|
MP
|
U
|
N
|
90.0
|
CT
|
11.2
|
3
|
26.0
|
3
|
|
21
|
19
|
F
|
12
|
10.0
|
P
|
C
|
Y
|
72.0
|
SR
|
15.0
|
3
|
25.0
|
3
|
|
22
|
14
|
M
|
8
|
10.0
|
MP
|
C
|
N
|
45.0
|
CT
|
2.2
|
1
|
10.4
|
3
|
|
23
|
52
|
M
|
10
|
3.7
|
MA
|
U
|
N
|
70.0
|
CT
|
6.7
|
1
|
21.1
|
1
|
|
24
|
30
|
F
|
9
|
3.0
|
MP
|
U
|
N
|
65.0
|
CT
|
0.0
|
0
|
26.3
|
3
|
|
25
|
42
|
M
|
24
|
8.3
|
MP
|
C
|
Y
|
30.0
|
SR
|
1.5
|
3
|
35.8
|
1
|
|
26
|
27
|
F
|
24
|
4.0
|
MXA
|
C
|
Y
|
60.0
|
SR
|
18.6
|
1
|
33.2
|
1
|
|
27
|
30
|
F
|
6
|
3.8
|
MA
|
C
|
N
|
45.0
|
CT
|
0.0
|
0
|
0.0
|
0
|
|
28
|
20
|
M
|
10
|
3.6
|
MA
|
C
|
Y
|
96.0
|
SR
|
26.8
|
3
|
40.1
|
3
|
|
29
|
20
|
M
|
9
|
4.6
|
MP
|
C
|
Y
|
36.0
|
SR
|
30.0
|
3
|
41.0
|
0
|
|
30
|
20
|
F
|
11
|
6.4
|
MP
|
C
|
Y
|
24.0
|
SR
|
5.1
|
3
|
34.2
|
3
|
|
31
|
45
|
M
|
5
|
3.3
|
MXP
|
C
|
N
|
104.0
|
CT
|
0.0
|
0
|
0.0
|
0
|
|
32
|
35
|
M
|
7
|
2.6
|
MP
|
C
|
N
|
24.0
|
CT
|
0.0
|
0
|
15.0
|
0
|
|
33
|
38
|
F
|
12
|
10.0
|
MP
|
C
|
Y
|
24.0
|
CT
|
10.8
|
3
|
55.2
|
0
|
|
34
|
38
|
F
|
9
|
6.7
|
P
|
C
|
Y
|
60.0
|
CT
|
11.0
|
3
|
40.0
|
1
|
|
35
|
55
|
M
|
12
|
4.6
|
MP
|
C
|
Y
|
8.0
|
CT
|
24.2
|
1
|
20.0
|
3
|
|
36
|
45
|
M
|
10
|
3.1
|
MA
|
P
|
Y
|
60.0
|
SR
|
18.2
|
1
|
25.8
|
0
|
|
37
|
45
|
M
|
26
|
6.7
|
MP
|
C
|
Y
|
156.0
|
SR
|
22.0
|
1
|
42.3
|
0
|
|
38
|
67
|
F
|
9
|
4.1
|
MXA
|
P
|
N
|
65.0
|
CT
|
0.0
|
0
|
23.4
|
2
|
|
39
|
45
|
M
|
30
|
3.8
|
P
|
C
|
Y
|
79.0
|
SR
|
34.6
|
2
|
44.0
|
2
|
A: duration from the onset of symptoms to hospitalization (months); B: histopathological subtypes; C: duration from the initial removal of primary tumor to recurrence (months); F: female; MA: mandible anterior; U: unicystic; N: no; CT: conservative therapy; M: male; MP: mandible posterior; C: conventional;
MXP: maxilla posterior; SR: surgical resection; Y: yes; P: peripheral; MXA: maxilla anterior.
The majority (n = 25; 62.5%) of ameloblastoma cases were of the conventional type followed by the unicystic type (n = 13; 32.5%). Of all the conventional types, the majority (n = 11; 44.0%) were of the follicular type. There were only two cases of the peripheral type (5.0%) [Table 1].
The majority (n = 26; 65.0%) of patients were treated using conservative therapy and the remaining (n = 14; 35.0%) underwent segmental resection followed by reconstruction of the operated sites of the jaw bones with non-vascularized bone graft and titanium reconstruction plates. Postoperative complications were reported in seven (17.5%) patients which consisted of site surgical infections (n = 5; 12.5%) and facial deformity (n = 2; 5.0%). None of the patients had reconstruction plate exposure as a complication. The mean follow-up duration of the patients was 55.4±2.9 months. Recurrence was reported among 19 (47.5%) patients. The first patient developed recurrence after eight months following initial removal of the primary tumor and the last patient had recurrence after 13 years (156 months). None of the patients died until the end of the follow-up time [Table 1].
Table 2 presents the association of clinicopathological characteristics with the expression of Ki-67. Expression of Ki-67 was observed in 21 (52.5%) cases with a mean expression of 13.3±1.2% (range = 0–34.8%). Of all the cases that expressed Ki-67, eight (38.1%) stained weakly, three (14.3%) stained moderately, and 10 (47.6%) stained strongly [Figure 1]. The mean expression of Ki-67 for cases with recurrence was significantly higher (23.4±8.6%) than that of non-recurrent cases (4.2±5.5%; p < 0.001). We found that there was a significant increase in mean expression of Ki-67 whereby patients with a tumor size equal to or greater than the mean tumor size had higher
mean expression (21.2±10.5%) than those with tumor size less than the mean tumor size (10.0±11.1%; p = 0.005).
Table 2: Association of clinical and pathological factors with Ki-67 expression (N = 40).
|
Age, years
|
|
|
|
0.083
|
|
≤ 50
|
23
|
10.1 ± 10.3
|
5.598–14.540
|
|
|
> 50
|
17
|
17.8 ± 12.9
|
11.143–24.357
|
|
|
Sex
|
|
|
|
0.830
|
|
Male
|
23
|
13.0 ± 12.1
|
7.759–18.195
|
|
|
Female
|
17
|
13.8 ± 12.1
|
7.569–20.059
|
|
|
Anatomical location
|
|
|
|
0.076
|
|
Posterior mandible
|
22
|
12.3 ± 11.3
|
7.301–17.351
|
|
|
Anterior mandible
|
9
|
12.9 ± 13.1
|
2.858–23.022
|
|
|
Posterior maxilla
|
4
|
8.5 ± 7.3
|
3.122–20.122
|
|
|
Anterior maxilla
|
2
|
9.3 ± 13.2
|
108.868–127.468
|
|
|
Palate
|
3
|
31.0 ± 6.1
|
15.902–46.195
|
|
|
Histological subtypes
|
|
|
|
0.097
|
|
Unicystic
|
18
|
17.5 ± 13.9
|
10.661–24.373
|
|
|
Peripheral
|
9
|
5.2 ± 5.8
|
0.764–9.676
|
|
|
Conventional
|
13
|
13.2 ± 9.8
|
7.206–19.106
|
|
|
Surgical approach
|
|
|
|
0.063
|
|
Conservative therapy
|
26
|
16.6 ± 5.6
|
4.349–12.853
|
|
|
Segmental resection
|
14
|
22.1 ± 9.3
|
16.789–27.490
|
|
|
Tumor size, cm
|
|
|
|
0.005
|
|
≤ 4.8 ± 2.2
|
28
|
10.0 ± 11.1
|
5.686–14.272
|
|
|
> 4.8 ± 2.2
|
12
|
21.2 ± 10.5
|
14.492–27.827
|
|
|
Lag period, months
|
|
|
|
0.071
|
|
≤ 9.2 ± 6.9
|
21
|
11.8 ± 8.2
|
2.548–10.061
|
|
|
> 9.2 ± 6.9
|
19
|
21.1 ± 10.6
|
16.013–26.241
|
|
|
Recurrence status
|
|
|
|
< 0.001
|
|
Recurrent
|
19
|
23.4 ± 8.6
|
1.750–6.733
|
|
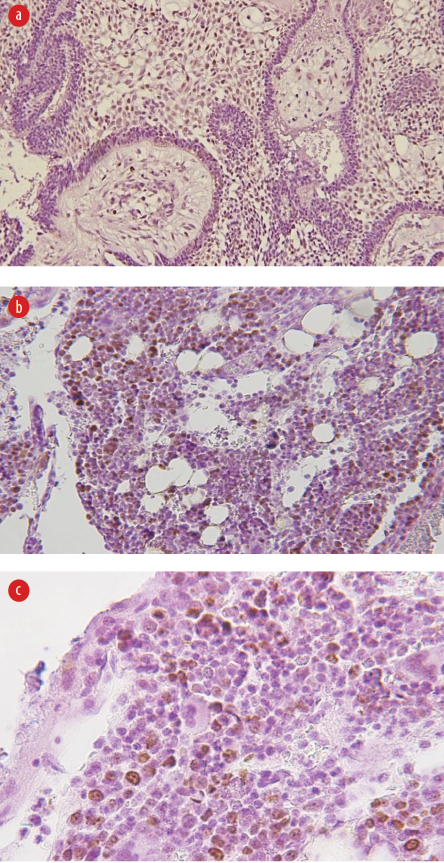 Figure 1: (a) Weak intranuclear staining for anti-Ki-67 for the case of follicular ameloblastoma (magnification = 100 ×), (b) moderate intranuclear staining for anti-Ki-67 for the case of peripheral ameloblastoma (magnification = 100 ×), and
Figure 1: (a) Weak intranuclear staining for anti-Ki-67 for the case of follicular ameloblastoma (magnification = 100 ×), (b) moderate intranuclear staining for anti-Ki-67 for the case of peripheral ameloblastoma (magnification = 100 ×), and
(c) strong intranuclear staining for anti-Ki-67 for plexiform ameloblastoma (magnification = 200 ×).
Although the mean expression of Ki-67 for older patients, patients that underwent segment resection, lag period above mean lag duration, and those with unicystic ameloblastoma was higher than their counter parts, but the difference did not reach statistical significance.
Table 3 presents the association of clinicopathological characteristics with expression of p53. Expression of p53 was found in 34 (85.0%) cases. The mean expression of p53 was 22.5±2.0% (range = 0–79.5.0%). Weak, moderate, and strong nuclear staining was found in eight (23.5%), 14 (41.2%), and 12 (35.3%) cases, respectively [Figure 2]. The mean expression of p53 for recurrent (32.5±18.3%) cases was significantly higher than that of non-recurrent cases (13.5±18.1%; p = 0.002). There was significantly higher expression of p53 for cases with tumor sizes larger than the mean tumor size (32.4±21.5%) compared to cases whose tumor size was smaller than the mean tumor size (12.3±18.6%). There was a positive association (p = 0.041) between increased mean expression of p53 for patients aged > 50 years (24.2±22.7%) and those aged ≤ 50 years (21.2±18.8%). The mean expression of p53 for females (24.4±21.8%) was higher than that of males (21.1±19.5%) but the difference was not significant (p = 0.615). Patients who were treated by segmental resection had a higher mean expression of p53 (32.2±19.8%) compared with patients treated conservatively (17.3±19.0%), but the difference was not significant (p = 0.076).
Table 3: Association of clinical and pathological factors with p53 expression.
|
Age, years
|
|
|
|
0.041
|
|
≤ 50
|
23
|
21.2 ± 18.8
|
13.445–29.421
|
|
|
> 50
|
17
|
24.2 ± 22.7
|
12.503–35.877
|
|
|
Sex
|
|
|
|
0.615
|
|
Male
|
23
|
21.1 ± 19.5
|
12.651–29.551
|
|
|
Female
|
17
|
24.4 ± 21.8
|
12.205–35.665
|
|
|
Anatomical location
|
0.060
|
|
Posterior mandible
|
22
|
20.9 ± 16.7
|
13.841–28.282
|
|
|
Anterior mandible
|
9
|
21.7 ± 25.2
|
2.239–41.029
|
|
|
Posterior maxilla
|
4
|
20.1 ± 37.6
|
-39.693–79.938
|
|
|
Anterior maxilla
|
2
|
28.3 ± 6.9
|
-33.960–90.580
|
|
|
Palate
|
3
|
36.3 ± 10.0
|
11.451–61.216
|
|
|
Histological subtypes
|
0.094
|
|
Unicystic
|
18
|
24.2 ± 17.0
|
15.689–32.617
|
|
|
Peripheral
|
9
|
15.3 ± 24.8
|
3.704–34.390
|
|
|
Conventional
|
13
|
25.2 ± 21.8
|
12.051–38.391
|
|
|
Surgical approach
|
0.076
|
|
Conservative therapy
|
26
|
17.3 ± 19.0
|
9.635–24.999
|
|
|
Segmental resection
|
14
|
32.2 ± 19.8
|
20.750–43.601
|
|
|
Tumor size, cm
|
0.043
|
|
≤ 4.8 ± 2.2
|
18
|
12.3 ± 18.6
|
11.057–25.511
|
|
|
> 4.8 ± 2.2
|
12
|
32.4 ± 21.5
|
18.725–46.066
|
|
|
Lag period, months
|
0.065
|
|
≤ 3
|
21
|
31.2 ± 18.9
|
5.618–22.839
|
|
|
> 3
|
19
|
14.7 ± 18.2
|
22.918–40.441
|
|
|
Recurrence status
|
0.002
|
|
Non-recurrent
|
21
|
13.5 ± 18.1
|
5.290–21.774
|
|
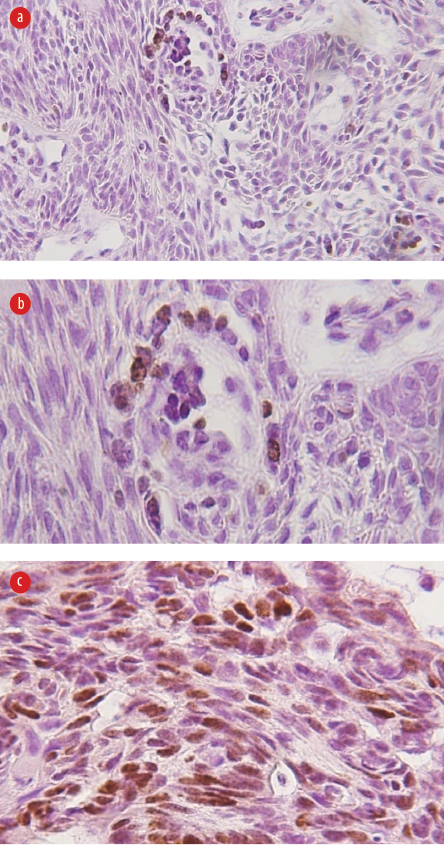 Figure 2: (a) Weak intranuclear staining for p53 protein for the case of peripheral ameloblastoma (magnification = 100 ×), (b) moderate intranuclear staining for p53 protein for the case of basal ameloblastoma (magnification = 100 ×), and
Figure 2: (a) Weak intranuclear staining for p53 protein for the case of peripheral ameloblastoma (magnification = 100 ×), (b) moderate intranuclear staining for p53 protein for the case of basal ameloblastoma (magnification = 100 ×), and
(c) strong intranuclear staining for p53 protein for plexiform ameloblastoma (magnification = 200 ×).
Table 4 shows the association of clinicopathological factors with recurrence among patients. There were significantly more cases with high p53 LI that had recurrence compared with low p53 LI (p = 0.041). In addition, it was found that there were significantly more cases with tumor size ≥ 5 cm that had recurrence compared with cases with tumor size < 5 cm (p < 0.001). Furthermore, there were significantly more cases with recurrence that were treated with segmental resection compared to those treated by conservative surgery (p < 0.001). Additionally, we observed that there were more conventional histological subtypes with recurrence than other histological subtypes with recurrence (p = 0.024).
Table 4: Association of clinical and pathological factors with recurrence (n = 40).
|
Age, years
|
|
|
0.061
|
|
≤ 35
|
8 (34.8)
|
15 (65.2)
|
|
|
> 35
|
11 (64.7)
|
6 (35.3)
|
|
|
Sex
|
|
|
0.218
|
|
Male
|
9 (39.1)
|
14 (60.9)
|
|
|
Female
|
10 (58.8)
|
7 (41.2)
|
|
|
Anatomical location
|
0.379*
|
|
Posterior mandible
|
10 (45.5)
|
12 (54.5)
|
|
|
Anterior mandible
|
4 (44.4)
|
5 (55.6)
|
|
|
Posterior maxilla
|
1 (25.0)
|
3 (75.0)
|
|
|
Anterior maxilla
|
1 (50.0)
|
1 (50.0)
|
|
|
Palate
|
3 (100.0)
|
0 (0.0)
|
|
|
Histological subtypes
|
0.024*
|
|
Unicystic
|
1 (11.1)
|
8 (88.9)
|
|
|
Peripheral
|
6 (46.2)
|
7 (53.8)
|
|
|
Conventional
|
12 (66.7)
|
6 (33.3)
|
|
|
Surgical approach
|
|
|
< 0.001*
|
|
Conservative therapy
|
5 (19.2)
|
21 (80.8)
|
|
|
Segmental resection
|
14 (100.0)
|
0 (0.0)
|
|
|
Tumor size, cm
|
|
|
< 0.001*
|
|
≤ 5
|
8 (28.6)
|
20 (71.4)
|
|
|
> 5
|
11 (91.7)
|
1 (8.3)
|
|
|
Lag period, months
|
|
|
0.074*
|
|
≤ 3
|
16 (84.2)
|
3 (15.8)
|
|
|
> 3
|
3 (14.3)
|
18 (85.7)
|
|
|
Ki67 expression
|
|
|
0.061
|
|
Low
|
8 (34.8)
|
15 (65.2)
|
|
|
High
|
11 (64.7)
|
6 (35.3)
|
|
|
p53 expression
|
|
|
0.041*
|
|
Low
|
4 (26.7)
|
11 (73.3)
|
|
There were more cases with high Ki-67 LI that had recurrence compared to cases with low Ki-67 LI with recurrence (p = 0.061). Similarly, patients aged > 35 years showed more rate of recurrence compared to younger patients although the difference was not significant (p = 0.061).
Discussion
We used the IHC method and sections from formalin-fixed paraffin-embedded tissue blocks of patients who were confirmed histologically to have ameloblastoma, treated, and followed-up for seve years. We assessed the clinicopathological factors associated with the expression of Ki-67 and p53 and the association of clinicopathological factors with recurrence.
We found the expression of p53 to be higher than that of Ki-67 (85.0% vs. 52.5%). Recurrence and larger tumor size than the mean tumor size were the independent factors significantly associated with both Ki-67 and p53 expression.
The expression of Ki-67 using the IHC method in ameloblastoma varies in different studies. This can be evidenced, for example, by the percentage of Ki-67 LI in this study, which was lower than the rate of expression reported in other studies. Previous studies have reported Ki-67 LI ranging from 82.3% to 100% in tissue blocks of patients with ameloblastoma.6,10,16 Some factors may explain the difference in the expression of Ki-67. For instance, it has been shown that cellular proliferation is subject to mutation of the cells. Therefore, the variation in the level of mutations for patients with ameloblastoma may explain the difference in the Ki-67 LI observed.20,21 Migaldi et al,5 found a positive association between microsatellite instabilities and increased proliferation of tumor cells detected by cellular proliferation biomarkers, including Ki-67. Furthermore, the difference of the methodological approach in scoring Ki-67 LI in terms of cut-off due to lack of universal cut-off standards, may also explain the difference in the level of expression of Ki-67 observed in various studies.
The mean Ki-67 LI in this study was associated with the recurrence and tumor size. Although the association of recurrence and Ki-67 expression contradicts reported studies, there is significant evidence of high Ki-67 LI with the possibility of ameloblastoma recurrence.22,23 Despite the lack of association, the mean percentage of Ki-67 LI in the present study was almost five times for the cases with recurrence compared with non-recurrent cases. In 2015, Ahlem et al,24 reported a positive association of Ki-67 LI with recurrence. The percentage of cases with high Ki-67 LI that had recurrence was significantly different from low Ki-67 LI cases that had recurrence. However, Carreón-Burciaga et al,11 reported contradicting findings in their study, in which the mean Ki-67 LI for non-recurrent cases was higher (15.7±13.6%) than that of recurrent cases (10.6±4.5%), but the difference was not significant (p > 0.05). Also, there was a significant association between tumor size and level of Ki-67 LI, which is different from the findings of two other studies that found no association between tumor size and Ki-67 LI among patients with ameloblastoma.6,16 Increased cell proliferation which is driven by mutation, usually leads to increased tumor size.25 This may help in explaining the fact that ameloblastoma cases with large tumor sizes are more likely to demonstrate high Ki-67 LI.
Despite few studies that have shown low p53 LI in patients with ameloblastoma, the vast majority of studies have reported high p53 LI. For example, Barboza et al,26 and Gadbail et al,6 showed 100% of p53 LI among patients with ameloblastoma. This indicates that expression of p53 is not only associated with malignant transformation but also increased aggressiveness and also in locally invasive tumors including ameloblastoma.27 This is in agreement with our findings. A similar observation was also reported in the study of Florescu et al,18 in 2012 who observed that an increased tendency of p53 expression was found more in recurrent cases than in non-recurrent cases, although the proportion of expression of the protein was low (52.9%). Evidence of p53 gene mutation was also reported in the study by Sharifi-Sistani et al,21 in which detection of gene mutation was done using PCR.28Additionally, there was a positive correlation between the expression of p53 with age, in which high p53 LI was found more in elderly patients than younger patients (p = 0.041). This may be due to aging being associated with an accumulation of various forms of mutations including those of the p53 gene.29
Furthermore, there was a positive association between tumor size and recurrence in this study. Cases with a larger tumor size than the mean tumor size had a higher recurrence rate than cases with a smaller tumor size than the mean tumor size. Although the association between recurrence and tumor size seems to be contracting, a positive association of the two variables has also been reported in another study.30 Another study found no association between tumor size and ameloblastoma recurrence.31
There was a significant association between conservative surgery with increased recurrence rate in this study, similar to previous studies.30–32 One systematic review study reported that surgical margin for patients with ameloblastoma ranging from 2.3–8 mm confers better prevention of recurrence.33 It was also argued that the use of intraoperative computed tomography scan to assess for adequacy of the surgical margin would help to prevent or reduce high chance of recurrence.
Furthermore, it was observed that there was a significant association between conventional subtypes (follicular) of ameloblastoma and recurrence. However, there is contradicting information regarding the association of recurrence with histological variants of ameloblastoma.34 Other studies have also reported a significant association of the follicular variant of ameloblastoma with recurrence.33,35,36 However, Au et al,32 and Bi et al,35 found no association between follicular type and increased risk of recurrence. The difference in the criteria used to classify the various histological types in different studies may help to explain the observed discrepancy. Additionally, lack of agreement across studies reported previously may in one way or another justify the lack of clinical association despite the statistical association that has been reported in some studies.
The prognostic role of the p53 protein in ameloblastoma has been studied extensively and evidence has shown that there is derangement in the p53 gene and even its product both for the benign and malignant transformed forms of ameloblastoma.37 Recurrence in ameloblastoma indicates local aggressiveness of the tumor and it has been associated with mutation of the p53 gene.26 In this study, a significant positive association between increased expression of p53 protein and increased risk of recurrence was also found.
The strength of this study is based on the use of two potential biomarkers to study the biological behaviors of tumors. However, the study faced some methodological limitations, including a small sample size due to limited funds to purchase enough primary antibodies for detecting Ki-67 and p53, which would have helped us to stain many cases. This reduced the power of the study and weakened the strength of the conclusions. In addition, the inability to perform molecular tests, e.g., p53 gene mutation due to lack of funds, contributed to the failure to have enough evidence regarding the link between the recurrence of ameloblastoma and expression of the biomarkers.
Conclusion
Although the expression of both Ki-67 and p53 in this study was relatively low, their expression can predict the possibility of ameloblastoma recurrence, particularly p53. Further studies with larger sample sizes involving survival and molecular analysis of these biomarkers would help explain their role in determining the recurrence of ameloblastoma.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
References
- 1. Bwambale P, Yahaya JJ, Owor G, Wabinga H. Histopathological patterns and biological characteristics of ameloblastoma: a retrospective cross-sectional study. J Taibah Univ Med Sci 2021 Nov;17(1):96-104.
- 2. Dandriyal R, Gupta A, Pant S, Baweja HH. Surgical management of ameloblastoma: conservative or radical approach. Natl J Maxillofac Surg 2011 Jan;2(1):22-27.
- 3. Ong’uti MN, Cruchley AT, Howells GL, Williams DM. Ki-67 antigen in ameloblastomas: correlation with clinical and histological parameters in 54 cases from Kenya. Int J Oral Maxillofac Surg 1997 Oct;26(5):376-379.
- 4. Amsi PT, Yahaya JJ, Kalungi S, Odida M. Immunohistochemical expression of BRCA1 and BRCA2 in a cohort of Ugandan men with prostate cancer: an analytical cross-sectional study. Afr J Urol 2020;26:1-9.
- 5. Migaldi M, Sartori G, Rossi G, Cittadini A, Sgambato A. Tumor cell proliferation and microsatellite alterations in human ameloblastoma. Oral Oncol 2008 Jan;44(1):50-60.
- 6. Gadbail AR, Patil R, Chaudhary M. Co-expression of Ki-67 and p53 protein in ameloblastoma and keratocystic odontogenic tumor. Acta Odontol Scand 2012 Dec;70(6):529-535.
- 7. Sun X, Bizhanova A, Matheson TD, Yu J, Zhu LJ, Kaufman PD. Ki-67 contributes to normal cell cycle progression and inactive X heterochromatin in p21 checkpoint-proficient human cells. Molecular and cellular biology 2017 Sep 1;37(17):e00569-16.
- 8. Shields CL, Shields JA. Basic understanding of current classification and management of retinoblastoma. Curr Opin Ophthalmol 2006 Jun;17(3):228-234.
- 9. Sathi GA, Tamamura R, Tsujigiwa H, Katase N, Lefeuvre M, Siar CH, et al. Analysis of immunoexpression of common cancer stem cell markers in ameloblastoma. Exp Ther Med 2012 Mar;3(3):397-402.
- 10. Lee SK, Kim YS. Current concepts and occurrence of epithelial odontogenic tumors: I. Ameloblastoma and adenomatoid odontogenic tumor. Korean J Pathol 2013 Jun;47(3):191-202.
- 11. Carreón-Burciaga RG, González-González R, Molina-Frechero N, Bologna-Molina R. Immunoexpression of Ki-67, MCM2, and MCM3 in ameloblastoma and ameloblastic carcinoma and their correlations with clinical and histopathological patterns. Dis Markers 2015;2015:683087.
- 12. Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev 2012 Jun;26(12):1268-1286.
- 13. Fayad DW. Immunohistochemical expression of P53 in different types of ameloblastoma conclusion. Al Anbar Univ 2010;8(1):66-73.
- 14. Hirayama T, Hamada T, Hasui K, Semba I, Murata F, Sugihara K. Immunohistochemical analysis of cell proliferation and suppression of ameloblastoma with special reference to plexiform and follicular ameloblastoma. Acta Histochemica et Cytochemica 2004;37(6):391-398.
- 15. Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G; STROCSS Group. STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery. Int J Surg 2019 Dec;72:156-165.
- 16. Wright JM, Soluk-Tekkeşin M. Odontogenic tumors: where are we in 2017? Odontojen Tümörler: 2017 Yılında Neredeyiz? J Istanb Univ Fac Dent 2017 Dec 2;51(3 Suppl 1):S10-S30.
- 17. Cadavid AM, Araujo JP, Coutinho-Camillo CM, Bologna S, Junior CA, Lourenço SV. Ameloblastomas: current aspects of the new WHO classification in an analysis of 136 cases. Surgical and Experimental Pathology 2019 Dec;2(1):1-6.
- 18. Florescu A, Simionescu C, Ciurea R, Pitru A. P53, Bcl-2 and Ki67 immunoexpression in follicular solid ameloblastomas. Rom J Morphol Embryol 2012;53(1):105-109.
- 19. Bologna-Molina R, Mosqueda-Taylor A, Lopez-Corella E, Almeida OP, Carrasco-Daza D, Garcia-Vazquez F, et al. Syndecan-1 (CD138) and Ki-67 expression in different subtypes of ameloblastomas. Oral Oncol 2008 Aug;44(8):805-811.
- 20. Abdel-Aziz A, Amin MM. EGFR, CD10 and proliferation marker Ki67 expression in ameloblastoma: possible role in local recurrence. Diagn Pathol 2012 Feb;7:14.
- 21. Sharifi-Sistani N, Zartab H, Babakoohi S, Saghravanian N, Jamshidi S, Esmaili H, et al. Immunohistochemical comparison of the expression of p53 and MDM2 proteins in ameloblastomas and keratocystic odontogenic tumors. J Craniofac Surg 2011 Sep;22(5):1652-1656.
- 22. Viner-Breuer R, Yilmaz A, Benvenisty N, Goldberg M. The essentiality landscape of cell cycle related genes in human pluripotent and cancer cells. Cell Div 2019 Dec;14:15.
- 23. Levine MS, Holland AJ. The impact of mitotic errors on cell proliferation and tumorigenesis. Genes Dev 2018 May;32(9-10):620-638.
- 24. Ahlem B, Wided A, Amani L, Nadia Z, Amira A, Faten F. Study of Ki67 and CD10 expression as predictive factors of recurrence of ameloblastoma. Eur Ann Otorhinolaryngol Head Neck Dis 2015 Nov;132(5):275-279.
- 25. Kufe DW, Pollock RE, Weichselbaum RR, Bast RC, Gansler TS, Holland JF, et al, editors. Holland-Frei cancer medicine. Hamilton (ON): BC Decker; 2003.
- 26. Barboza CA, Pereira Pinto L, Freitas RdeA, Costa AdeL, Souza LB. Proliferating cell nuclear antigen (PCNA) and p53 protein expression in ameloblastoma and adenomatoid odontogenic tumor. Braz Dent J 2005;16(1):56-61.
- 27. Zhong Y, Guo W, Wang L, Chen X. Molecular markers of tumor invasiveness in ameloblastoma: an update. Ann Maxillofac Surg 2011 Jul;1(2):145-149.
- 28. Al-Salihi K, Li LY, Azlina A. P53 gene mutation and protein expression in ameloblastomas. Braz J Oral Sci 2006;5(17):1034-1040.
- 29. Richardson RB. p53 mutations associated with aging-related rise in cancer incidence rates. Cell Cycle 2013 Aug;12(15):2468-2478.
- 30. Yang R, Liu Z, Gokavarapu S, Peng C, Ji T, Cao W. Recurrence and cancerization of ameloblastoma: multivariate analysis of 87 recurrent craniofacial ameloblastoma to assess risk factors associated with early recurrence and secondary ameloblastic carcinoma. Chinese Journal of Cancer Research 2017 Jun;29(3):189-195.
- 31. Fregnani ER, da Cruz Perez DE, de Almeida OP, Kowalski LP, Soares FA, de Abreu Alves F. Clinicopathological study and treatment outcomes of 121 cases of ameloblastomas. Int J Oral Maxillofac Surg 2010 Feb;39(2):145-149.
- 32. Au SW, Li KY, Choi WS, Su YX. Risk factors for recurrence of ameloblastoma: a long-term follow-up retrospective study. Int J Oral Maxillofac Surg 2019 Oct;48(10):1300-1306.
- 33. De Silva I, Rozen WM, Ramakrishnan A, Mirkazemi M, Baillieu C, Ptasznik R, et al. Achieving adequate margins in ameloblastoma resection: the role for intra-operative specimen imaging. Clinical report and systematic review. PLoS One 2012;7(10):e47897.
- 34. Ajila V, Hegde S. Ameloblastomas vs recurrent ameloblastomas: a systematic review. J Oral Med Oral Surg 2022;28(1):1-8.
- 35. Bi L, Wei D, Hong D, Wang J, Qian K, Wang H, et al. A retrospective study of 158 cases on the risk factors for recurrence in ameloblastoma. Int J Med Sci 2021 Jul;18(14):3326-3332.
- 36. Milman T, Ying GS, Pan W, LiVolsi V. Ameloblastoma: 25 year experience at a single institution. Head Neck Pathol 2016 Dec;10(4):513-520.
- 37. Kumamoto H, Izutsu T, Ohki K, Takahashi N, Ooya K. p53 gene status and expression of p53, MDM2, and p14ARF proteins in ameloblastomas. Journal of Oral Pathology & Medicine 2004 May;33(5):292-299.