Kidney disease is the eighth leading cause of death in Oman, responsible for around 16.9 deaths per 100 000 resident population in 2021.1,2 Among kidney diseases, chronic kidney disease (CKD) stands out as a globally significant cause of morbidity and mortality.3
According to the Kidney Disease Improving Global Outcome 2012 guidelines, CKD can be classified based on the cause, glomerular filtration rate (GFR) category, and albuminuria category. There are five stages of CKD based on GFR (mL/min/1.73 m2) levels: stage 1 (GFR ≥ 90), stage 2 (GFR = 60–89), stage 3a (GFR = 45–59), stage 3b (GFR = 30–44), stage 4 (GFR = 15–29), and stage 5 (GFR < 15).4
GFR is considered the best marker for kidney function. It is a measure of the quantity of plasma the kidneys filter in one minute,4 GFR can be measured only indirectly5 using either measured GFR (mGFR), which estimates the elimination rates of exogenous filtration markers such as inulin, iohexol, iothalamate, or chromium-EDTA, estimated GFR (eGFR), or serum levels of endogenous filtration markers such as cystatin C or creatinine. GFR measured using clearance methods, though informative, is less accurate and should be interpreted with caution, and is rarely used in clinical settings due to practical difficulties, patient inconvenience, and expense.6–8
Serum creatinine remains the most routinely-used endogenous marker of kidney function worldwide. Creatinine is affected by several factors including age, sex, ethnicity, muscle mass, diet, and exercise.9,10 The Kidney Disease Improving Global Outcome 2012 recommends calculating eGFR using the CKD Epidemiology Collaboration (CKD-EPI 2009) equation, or with an alternative comparable eGFR equation. The serum creatinine measurement should be done using a specific assay with calibration traceable to the international standard reference materials with minimal bias compared to isotope dilution mass spectrometry reference methodology.4 Because of mGFR’s limitations, eGFR is preferred in clinical practice. Three eGFR equations have been developed in the past decades to assess the GFR. These are the Cockcroft-Gault equation (1976),11 the Modification of Diet in Renal Disease (MDRD) equation (1999),12 and the CKD-EPI equation (2009).13 The Cockcroft-Gault equation estimates the creatinine clearance (CrCl) based on weight, age, sex, and serum creatinine.11 However, the CrCl calculated using this equation may overestimate eGFR for overweight patients and those with fluid retention issues. In addition, using the Cockcroft-Gault equation with other methods of creatinine measurement might create bias.14,15 Consequently, the Cockcroft-Gault equation is rarely used in current clinical practice.16
The MDRD and CKD-EPI equations, on the other hand, estimate GFR (mL/min/1.73 m2) rather than CrCl and require serum creatinine, age, sex, and ethnicity but not weight, as the results are adjusted to the body surface area. The original MDRD equation was developed based on a sample of 1628 patients with CKD, and it contained a constant factor of 186 which was replaced later on in the re-expressed four-variable MDRD equation by a coefficient factor of 175, as the serum creatinine assay was standardized to the isotope dilution mass spectrometry reference method.12,17 The MDRD equation performed well in patients with lower levels of GFR (≤ 60 mL/min/1.73 m2), but it underestimated GFR at higher GFR values.12 This called for a new equation to use in patients with higher GFR. In 2009, Stevens et al,13 derived the CKD-EPI formula with a lower bias for eGFR > 60 mL/min/1.73 m2, which led to a 1.6% lower estimated CKD prevalence in the USA population compared to the older MDRD method. Just like MDRD, the CKD-EPI formula relied on age, sex, and race as surrogates for non-GFR determinants of serum creatinine. Interestingly, while the MDRD equation yielded higher prevalence estimates of CKD in women, Caucasian, and the elderly, the CKD-EPI equation showed reduced prevalence in women and Caucasian, but not in the elderly.13
Many laboratories around the globe embraced the change from MDRD to the CKD-EPI equation. Several studies highlighted the effects of CKD-EPI implementation in changing the CKD stages in a significant portion of patients into higher eGFR stages.18–21 When compared to a gold standard method of GFR measurement, the CKD-EPI equation was also found in several studies to be the most accurate, precise, and least biased among other eGFR equations.22,23 In Oman, a study by Al Maqbali et al,24 was conducted in 2013 to compare MDRD186, MDRD175, and CKD-EPI in the Omani diabetic population. It was shown that the performance of MDRD186 was comparable to CKD-EPI, whereas MDRD175 was found to underestimate GFR, and hence increase the prevalence of CKD in the diabetic population.24 Therefore, the current healthcare system continues to use the conventional MDRD186 for GFR estimation.
No local validation study has been conducted in the Omani population to assess the performance of CKD-EPI and MDRD equations in comparison with the gold standard method of GFR measurement. Furthermore, it is not known whether implementing the CKD-EPI equation in the Omani population would have a significant impact on reclassification of CKD stages. Therefore, this study aimed to evaluate the performance (bias, precision, and accuracy) of MDRD and CKD-EPI equations in comparison to the gold standard method (technetium-99m diethylenetriaminepentaacetic acid (99Tc-DTPA renogram) using patient data from a tertiary hospital in Oman. It also aimed to correlate the performance of MDRD and CKD-EPI equations on the reclassification of CKD stages in Omani adult patients.
Methods
This cross-sectional study was approved by the ethical committee at the Royal Hospital, Muscat (Ref. SRC#91/2021). We recruited two groups of CKD patients aged 18–70 years treated at the Royal Hospital from January to October 2021. The first group included 48 patients who underwent a 99Tc-DTPA renogram procedure for GFR measurement. The second group comprised 30 348 adult CKD patients. Patients aged < 18 years, > 70 years, and pregnant women were excluded. Serum creatinine data of the included patients were analyzed. The following equations were used:
MDRD equations
- MDRD186 equation: eGFR (mL/min/1.73 m2) = 186 (S.Cr in µmol/L × 0.011312)-1.154 × (age)-0.203 × (0.742 if female) × (1.212 if African American/Black)
- MDRD175 equation: eGFR (mL/min/1.73 m2) = 175 (S.Cr in µmol/L × 0.011312)- 1.154 × (age)-0.203 × (0.742 if female) × (1.212 if African American/Black)
CKD-EPI (2009) equations
- Female with Cr < 62 µmol/L: eGFR (mL/min/1.73 m2) = 144 × (Cr/61.6)-0.329 × (0.993)age
- Female with Cr > 62 µmol/L: eGFR (mL/min/1.73 m2) = 144 × (Cr/61.6)-1.209 × (0.993)age
- Male with Cr < 80 µmol/L: eGFR (mL/min/1.73 m2) = 141 × (Cr/79.2)-0.411 × (0.993)age
- Male with Cr > 80 µmol/L: eGFR (mL/min/1.73 m2) = 141 × (Cr/79.2)-1.209 × (0.993)age
The sample size for the comparison of MDRD and CKD-EPI equations to 99Tc-DTPA renogram was calculated using data from three studies from the literature,12,18,25 where the correlation between mGFR and eGFR was found to be 0.83–0.88. The proportional differences between the prevalence of different CKD stages (mainly stages 1 and 2) according to both MDRD and CKD-EPI equations were 27% and 36%, respectively. Hence, when using a sample calculator, it was found that a sample of 64 pairs was required to achieve a study power of 80% and a two-sided significance of 5% for detecting a difference of 0.11 between marginal proportions.
For data entry and analysis, Microsoft Office Excel 2019 was used. The prevalence of each CKD stage by both MDRD and CKD-EPI equations was calculated using pre-determined cut-off value to indicate the abnormal levels (taken from the international guidelines for each parameter). The number of abnormal results was divided by the population size in that group and then multiplied by 100 to yield the prevalence percentage. The comparison between both equations to a reference method was assessed using a Bland-Altman plot.
Accuracy was calculated as the percentage of eGFR within 30% of mGFR. Precision was calculated as the interquartile range (Q3-Q1). Bias was calculated as the mean of the bias percentage using the formula: (mGFR-eGFR by specified equation/mGFR) ×100.
Results
The first group (99Tc-DTPA renogram) comprised 48 participants with a mean age of 38±10.4 years, mostly (64.5%) were male. The median GFR (mL/min/1.73 m2) measured by 99Tc-DTPA renogram was 106.0 (range = 104.0–112.0), while the median eGFR values calculated by MDRD175, MDRD186, and CKD-EPI were 92.5 (81.8–104.1), 98.3 (86.9–110.6), and 102.1 (92.3–117.4), respectively [Table 1]. The second group, comprising 30 348 participants with a mean age of 43.2±13.6 years. This group had a female majority (58.4%). Their mean GFR values, as calculated by MDRD175, MDRD186, and CKD-EPI were 100.4±40.2, 106.7±42.7, and 99.5±29.2 mL/min/1.73 m2, respectively.
Table 1: The median, bias, precision, and accuracy of glomerular filtration rate (GFR) for the first group
of patients (N = 48) measured using 99Tc-DTPA renogram and calculated by MDRD175, MDRD186, and CKD-EPI.
|
Measured GFR (99Tc-DTPA renogram)
|
106.0 (104.0–112.0)
|
-
|
-
|
-
|
|
MDRD175
|
92.5 (81.8–104.1)
|
12.9
|
22.3
|
91.7
|
|
MDRD186
|
98.3 (86.9–110.6)
|
7.5
|
23.8
|
93.8
|
99Tc-DTPA: technetium-99m diethylenetriaminepentaacetic acid; MDRD: Modification of Diet in Renal Disease; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; P (30): percentage of estimated GFR within 30% of measured GFR.
The CKD-EPI showed the highest accuracy with a p(30) of 95.8%, and the least bias compared to the conventional and revised MDRD equations [Table 1]. However, the CKD-EPI equation also demonstrated the least precision compared to the MDRD equations. Table 1 shows the median, bias, precision, and accuracy of all equations in comparison with the reference method of GFR measurement.
The eGFR by both MDRD186, MDRD175, and CKD-EPI equations correlated moderately well with the measured GFR using 99Tc-DTPA renogram (r = 0.4, 0.4, and 0.5, respectively; p < 0.010), and all were within the accepted limits of agreement. Figure 1 shows the Bland-Altman plots of the three equations in comparison with the reference GFR measured by the 99Tc-DTPA renogram.
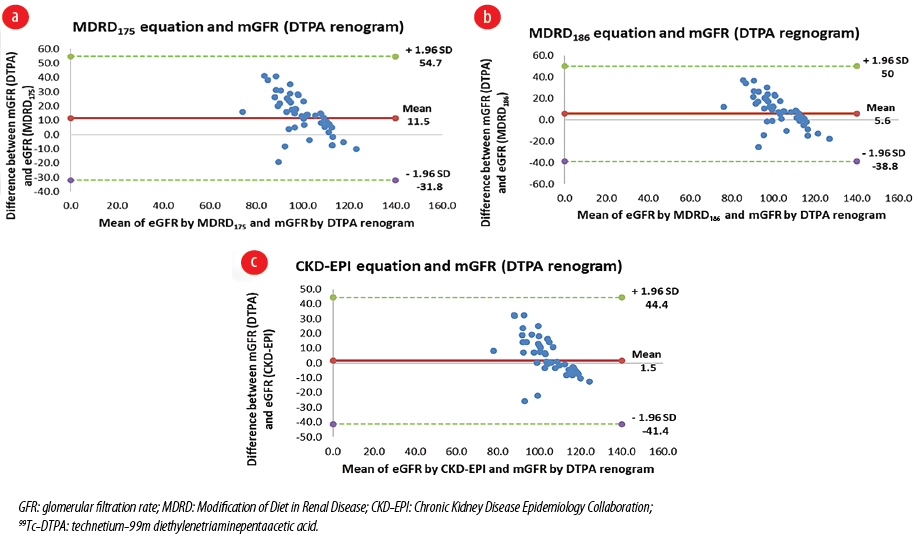 Figure 1: Bland-Altman plots comparing the calculated GFR with the measured GFR by 99Tc-DTPA renogram in the first group (N = 48). (a) MDRD175 equation with DTPA renogram, (b) MDRD186 equation with DTPA renogram, and (c) CKD-EPI equation with DTPA renogram.
Figure 1: Bland-Altman plots comparing the calculated GFR with the measured GFR by 99Tc-DTPA renogram in the first group (N = 48). (a) MDRD175 equation with DTPA renogram, (b) MDRD186 equation with DTPA renogram, and (c) CKD-EPI equation with DTPA renogram.
The MDRD and CKD-EPI equations remained in close agreement in GFR estimation at stages 3b, 4, and 5. However, the MDRD175 equation started deviating from the remaining two equations at stage 3a at around GFR of 50 mL/min/1.73 m2, while the MDRD186 started deviating from the CKD-EPI equation at stage 2 at around GFR of 69.0 mL/min/1.73 m2 [Figure 2].
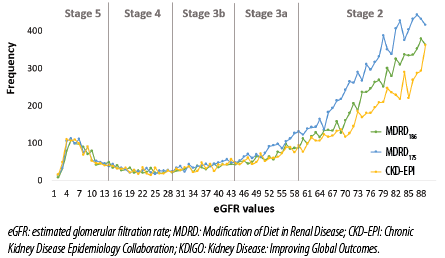 Figure 2: Distribution of eGFR results calculated by both MDRD and CKD-EPI equations and classified into KDIGO CKD stages in the second group (N = 30 348).
Figure 2: Distribution of eGFR results calculated by both MDRD and CKD-EPI equations and classified into KDIGO CKD stages in the second group (N = 30 348).
The reclassification of CKD stages based on the three equations showed that the eGFR values based on MDRD175 differed significantly from both MDRD186 and CKD-EPI at stages 1–3b, classifying more patients into stages 2–3b. The CKD-EPI equation yielded different CKD classification at stages 1 and 2 compared to the MDRD186 equation (p < 0.001) but had a similar performance at stages 3a–5, with the CKD-EPI equation classifying more patients into stage 1 [Figure 3].
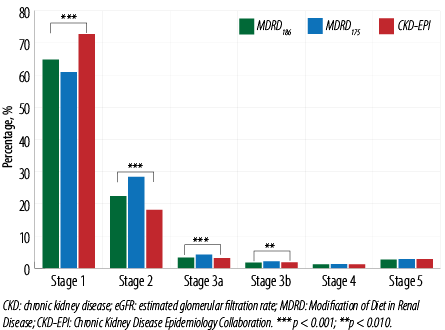 Figure 3: Distribution of CKD stages by eGFR calculated by MDRD175, MDRD186, and CKD-EPI equations in the second group.
Figure 3: Distribution of CKD stages by eGFR calculated by MDRD175, MDRD186, and CKD-EPI equations in the second group.
Discussion
This study, based on the Omani population, found the CKD-EPI equation to have higher accuracy and lower bias, but lower precision in estimating GFR compared to the conventional and revised MDRD equations. It also showed that the GFR estimated by the conventional MDRD equation deviated from the GFR estimated by the CKD-EPI equation at CKD stage 2, while the revised MDRD equation started deviating at CKD stage 3a. The use of the CKD-EPI equation classified more patients to a higher GFR stage, mainly CKD stage 1, as compared to the conventional and revised MDRD equations. However, it had a similar performance to the conventional MDRD equation at stages 3a–5.
The findings correlate with the recent study from Ireland, which compared CKD-EPI and revised MDRD equations in 300 000 samples of inpatients, outpatients, and general practice patients.18 Their results showed that the CKD-EPI equation performed better at high GFR levels, but the change in CKD reclassification occurred mostly from stages 2 to 1 and from 3a to 2. The study concluded that changing from MDRD to CKD-EPI will have little impact on most patients’ eGFR and CKD stages, however, for the rest of the patients it will reduce the number of cases identified as CKD and thus reduce unnecessary nephrology referrals.18 Similar conclusions were observed in the Kidney Early Evaluation Program in the USA19 and in another study by Korhonen et al.20
As for a closer Asian-based population, the comparison between both equations was studied in a multi-ethnic Malaysian population with a comparison to a gold standard method of GFR measurement, namely 51chromium EDTA plasma clearance. The study highlighted the superiority of the CKD-EPI equation in terms of accuracy and precision as compared to MDRD.22 Similar findings were obtained in a Saudi Arabian study that compared both equations to inulin clearance.23
In Oman, a study by Al Maqbali et al,24 compared the performances of MDRD186, MDRD175, and CKD-EPI in the Omani diabetic patients attending primary care, specifically looking at their CKD reclassification based on eGFR.24 The study concluded that the performance of MDRD186 and CKD-EPI was in accordance with each other and relayed comparable results. The study also demonstrated that compared with the aforementioned formulae, MDRD175 underestimated GFR, especially at stages 2–3, increasing CKD diagnosis.24 However, that study did not compare the performances with a gold standard GFR measurement method.
The CKD-EPI equation was originally developed based on a largely young-to-middle-aged population with a mean mGFR of 68 mL/min/1.73 m2. A study in the Netherlands looked into the consequences of introducing the CKD-EPI equation in older Northern Europeans.21 It has shown that the equation yielded higher GFR values in younger age groups and revealed a steeper GFR decline with aging as compared to the MDRD equation. Hence, younger people were classified more into higher GFR stages whereas older people, especially men, were into lower GFR stages.21 Similar were the findings of Al Maqbali et al,24 and again observed in the present study.
At the time of this study, the eGFR equation in use at the Royal Hospital, Oman was the MDRD186 equation. This study has confirmed the CKD-EPI equation to have better accuracy and lower bias than MDRD186 equation, raising the possibility that CKD-EPI might be preferred for CKD detection and classification among Omanis. It is worth noting that when the study was conducted, the CKD-EPI 2021 equation had just been released.26 Afterwards, our data was recalculated using the 2021 equation, and an agreement of 99.7% was found between the 2009 and 2021 CKD-EPI equations (data not shown).
Several studies discussed the change in the CKD-EPI 2021 equation as compared to its 2009 version. A study by Munch et al,27 compared eGFR calculated using CKD-EPI 2009 (assuming non-Black ethnicity; CKD-EPI09 - NB) and CKD-EPI 2021 with 51chromium EDTA clearance as the gold standard method of GFR measurement. The study concluded that, to a small degree, the CKD-EPI 2021 equation performed better compared to the CKD-EPI 2009 equation; however, the CKD-EPI 2009 was superior in terms of CKD stages reclassification at GFR < 60 mL/min/1.73 m2.27 Another study by Gansevoort et al,28 supported European nephrologists switching to the 2021 version of CKD-EPI equation, given its effect on reclassifying high-risk patients with diabetes or cardiovascular disease into lower risk categories. Similar findings were reported from a Spanish study that investigated the effect of changing to CKD-EPI 2021.29
Although our study was limited by its relatively small sample size of patients who underwent the reference GFR measurement method (99Tc-DTPA-renogram), and the fact that most of the patients included in the first group were healthy kidney donors who had GFRs or CKD 1–2 only, it was empowered by the inclusion of > 37 000 patients in the second group, who represented the general Omani population with different comorbidities and covering all CKD stages.
As a consequence of the outcome of our study, the Royal Hospital has substituted the previously used MDRD186 method with the CKD-EPI (2021) equation. Future prospective studies in such a population can further confirm the superiority and advantageousness of the CKD-EPI equation over the MDRD equation.
Conclusion
This study evaluated the performance of MDRD186, MDRD175, and CKD-EPI equations in comparison to 99Tc-DTPA renogram and correlated their impacts on reclassifying CKD stages in adult patients in Oman. It has shown that the CKD-EPI equation was the most accurate and the least biased in the general Omani population, with the differences in CKD reclassification appearing in CKD stages 1–2 only. Based on the findings of the study, the Royal Hospital has switched to the CKD-EPI equation from the previously used MDRD equations.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Al Alawi I, Al Salmi I, Al Mawali A, Al Maimani Y, Sayer JA. End-stage kidney failure in Oman: an analysis of registry data with an emphasis on congenital and inherited renal diseases. Int J Nephrol 2017;2017:6403985.
- 2. Al Ismaili F, Al Salmi I, Al Maimani Y, Metry AM, Al Marhoobi H, Hola A, et al. Epidemiological transition of end-stage kidney disease in Oman. Kidney Int Rep 2016 Sep;2(1):27-35.
- 3. Lamb EJ, Levey AS, Stevens PE. The kidney disease improving global outcomes (KDIGO) guideline update for chronic kidney disease: evolution not revolution. Clin Chem 2013 Mar;59(3):462-465.
- 4. Murton M, Goff-Leggett D, Bobrowska A, Garcia Sanchez JJ, James G, Wittbrodt E, et al. Burden of chronic kidney disease by KDIGO categories of glomerular filtration rate and albuminuria: a systematic review. Adv Ther 2021 Jan;38(1):180-200.
- 5. Levey AS, Grams ME, Inker LA. Uses of GFR and albuminuria level in acute and chronic kidney disease. N Engl J Med 2022 Jun;386(22):2120-2128.
- 6. Toffaletti JG, McDonnell EH. Variation of serum creatinine, cystatin C, and creatinine clearance tests in persons with normal renal function. Clin Chim Acta 2008 Sep;395(1-2):115-119.
- 7. Ebert N, Bevc S, Bökenkamp A, Gaillard F, Hornum M, Jager KJ, et al. Assessment of kidney function: clinical indications for measured GFR. Clin Kidney J 2021 Feb;14(8):1861-1870.
- 8. Mula-Abed WA, Al Rasadi K, Al-Riyami D. Estimated glomerular filtration rate (eGFR): a serum creatinine-based test for the detection of chronic kidney disease and its impact on clinical practice. Oman Med J 2012 Mar;27(2):108-113.
- 9. Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 2009 Nov;20(11):2305-2313.
- 10. Peake M, Whiting M. Measurement of serum creatinine–current status and future goals. Clin Biochem Rev 2006 Nov;27(4):173-184.
- 11. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16(1):31-41.
- 12. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999 Mar;130(6):461-470.
- 13. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009 May;150(9):604-612.
- 14. Schmidt RL, Straseski JA, Raphael KL, Adams AH, Lehman CM. A Risk assessment of the jaffe vs enzymatic method for creatinine measurement in an outpatient population. PLoS One 2015 Nov;10(11):e0143205.
- 15. Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, et al; National Kidney Disease Education Program Laboratory Working Group. Recommendations for improving serum creatinine measurement: a report from the laboratory working group of the national kidney disease education program. Clin Chem 2006 Jan;52(1):5-18.
- 16. Fernandez-Prado R, Castillo-Rodriguez E, Velez-Arribas FJ, Gracia-Iguacel C, Ortiz A. Creatinine clearance is not equal to glomerular filtration rate and cockcroft-gault equation is not equal to CKD-EPI collaboration equation. Am J Med 2016 Dec;129(12):1259-1263.
- 17. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006 Aug;145(4):247-254.
- 18. Reeve JL, Davis M, Twomey PJ. Observations from a teaching hospital in Ireland: changing from MDRD to CKD-EPI eGFR in routine practice. J Clin Pathol 2021 Sep;74(9):608-611.
- 19. Stevens LA, Li S, Kurella Tamura M, Chen SC, Vassalotti JA, Norris KC, et al. Comparison of the CKD epidemiology collaboration (CKD-EPI) and modification of diet in renal disease (MDRD) study equations: risk factors for and complications of CKD and mortality in the kidney early evaluation program (KEEP). Am J Kidney Dis 2011 Mar;57(3)(Suppl 2):S9-S16.
- 20. Korhonen PE, Kautiainen H, Järvenpää S, Kivelä SL. Time to change the glomerular filtration rate estimating formula in primary care? Eur J Intern Med 2012 Jun;23(4):355-357.
- 21. van den Brand JA, van Boekel GA, Willems HL, Kiemeney LA, den Heijer M, Wetzels JF. Introduction of the CKD-EPI equation to estimate glomerular filtration rate in a Caucasian population. Nephrol Dial Transplant 2011 Oct;26(10):3176-3181.
- 22. Jalalonmuhali M, Lim SK, Md Shah MN, Ng KP. MDRD vs. CKD-EPI in comparison to 51Chromium EDTA: a cross sectional study of Malaysian CKD cohort. BMC Nephrol 2017 Dec;18(1):363.
- 23. Al-Wakeel JS. Accuracy and precision of the CKD-EPI and MDRD predictive equations compared with glomerular filtration rate measured by inulin clearance in a Saudi population. Ann Saudi Med 2016;36(2):128-134.
- 24. Al-Maqbali SR, Mula-Abed WA. Comparison between three different equations for the estimation of glomerular filtration rate in Omani patients with type 2 diabetes mellitus. Sultan Qaboos Univ Med J 2014 May;14(2):e197-e203.
- 25. Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function–measured and estimated glomerular filtration rate. N Engl J Med 2006 Jun;354(23):2473-2483.
- 26. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021 Nov;385(19):1737-1749.
- 27. Munch PV, Heide-Jorgensen U, Jensen SK, Birn H, Vestergaard SV, Frokiaer J, et al. Performance of the race-free CKD-EPI creatinine-based eGFR equation in a Danish cohort with chromium-51-EDTA clearance measurements. Clin Kidney J 2023:sfad253.
- 28. Gansevoort RT, Anders HJ, Cozzolino M, Fliser D, Fouque D, Ortiz A, et al. What should European nephrology do with the new CKD-EPI equation? Nephrol Dial Transplant 2023 Jan;38(1):1-6.
- 29. Escribano-Serrano J, Jiménez-Varo E, Escribano-Cobalea M, López-Ceres A, Casto-Jarillo C, Hormigo-Pozo A, et al; Grupo de Estudio de Riesgo Vascular Alcalá (GERVA). Is the use of the new chronic kidney disease epidemiology consortium (CKD-EPI 2021) formula appropriate for the Spanish population? Rev Clin Esp (Barc) 2023 Mar;223(3):144-153.