Acute aortic dissection (AD) is exceedingly rare, occurring in approximately three out of 100 000 population per year.1 It is estimated that 40% of patients with acute AD die before reaching the hospital,2 while the in-hospital mortality is approximately 20%.3 Symptoms can be atypical and mimic other causes of chest pain such as acute coronary syndrome or pulmonary embolism, and classically include sudden chest pain characterized as tearing or ripping that may radiate into the back or scapular region.4 Physical examination may reveal blood pressure or pulse discrepancy between the right and left upper extremities. With the involvement of the coronary arteries, myocardial injury is a potential complication, and this may present with or without ST-segment elevation in the electrocardiogram (ECG), which can be a confounder.
Case Report
A 40-year-old male presented to the emergency department (ED) with chest pain that started one hour before arrival. The pain was pleuritic and associated with shortness of breath. The patient’s medical history included obesity, obstructive sleep apnea, hypertension, tobacco use, and right diaphragmatic palsy secondary to viral illness.
On arrival, he was alert and oriented, but in respiratory distress. Vital signs were temperature of 36.6 °C, heart rate of 123 beats per minute, blood pressure of 135/87 mmHg in the right arm and 123/66 mmHg in the left arm, breathing rate of 26 per minute, and oxygen saturation of 89% on room air, which improved to 94% on a non-rebreather mask. Physical examination revealed respiratory distress with diffuse expiratory wheezing. There was no cardiac murmur, jugular venous distention, pulse deficits, or focal neurologic deficits.
ECG revealed sinus tachycardia with an incomplete right bundle branch block and poor R-wave progression [Figure 1]. Bedside transthoracic echocardiography (TTE) demonstrated moderate pericardial effusion with > 30% mitral inflow variability with inspiration and inferior vena cava plethora with minimal respiratory variation [Figure 2]. Five months earlier, the patient had a TTE performed which revealed an ejection fraction of 65% and a dilated ascending aorta with a diameter of 5 cm. He denied any history of deep venous thrombosis or pulmonary embolism.
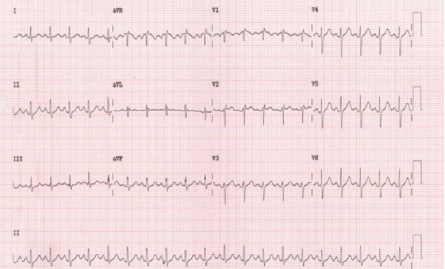 Figure 1: Twelve-lead electrocardiogram showing sinus tachycardia and an incomplete right bundle branch block.
Figure 1: Twelve-lead electrocardiogram showing sinus tachycardia and an incomplete right bundle branch block.
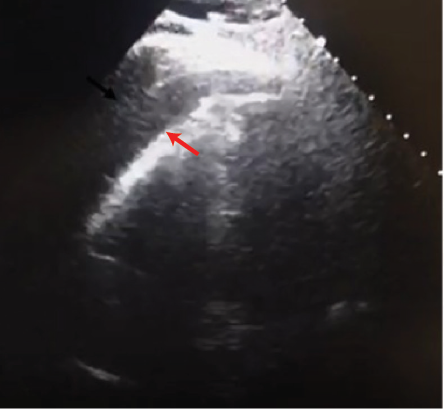 Figure 2: Transthoracic echocardiography (apical four-chamber view) demonstratingthe pericardial effusion (red arrow).
Figure 2: Transthoracic echocardiography (apical four-chamber view) demonstratingthe pericardial effusion (red arrow).
Laboratory tests revealed white blood cell count of 12 × 109 K/mcL (reference range = 4.5–11.0), hemoglobin of 17.1 g/dL (12.6–17.4), platelets of 190 K/mcL (153–367), creatinine of 1.3 mg/dL (0.66–1.25), D-dimer of 138 ng/mL (< 255), and initial troponin I of 0.017 ng/mL (< 0.03), followed by a second troponin-I 0.08 ng/mL five hours later. Computed tomography (CT) angiography of the chest demonstrated moderate-sized pericardial effusion (mean density of 39 Hounsfield units) consistent with hemopericardium with associated acute intramural hematoma of the ascending aorta, aortic arch, descending thoracic aorta, right and left coronary arteries, and left vertebral artery [Figure 3]. The aneurysmal dilation of the ascending aorta measured 5 cm at the level of the right main pulmonary artery. There was no intimal flap in the aortic lumen nor any filling defect in the main or segmental pulmonary arteries.
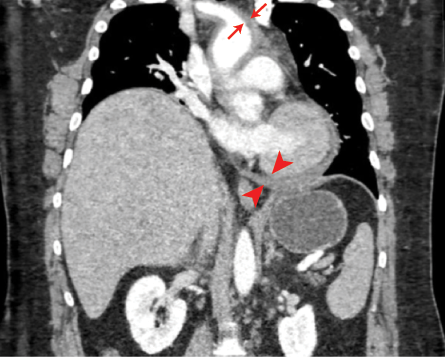 Figure 3: Coronal view of the CT angiography demonstrating the involvement of the ascending aorta, aortic arch, descending thoracic aorta, and right and left coronary arteries.
Figure 3: Coronal view of the CT angiography demonstrating the involvement of the ascending aorta, aortic arch, descending thoracic aorta, and right and left coronary arteries.
The patient was started on an esmolol infusion at 50 mcg/kg/min and was taken to the operating room for an ascending aortic repair. An aortic tear above the right coronary sinus was seen with a thrombosed false lumen and hemopericardium. The postoperative course was uncomplicated, and the patient was discharged five days later in a stable condition.
Discussion
Dissection into the pericardium with subsequent tamponade is one of the most common causes of death from AD.5 Another cause of mortality is dissection leading to aortic-free rupture into the pleural space or mediastinum.5 Our patient was hemodynamically stable while in the ED despite the presence of a moderate-sized pericardial effusion. Therefore, a pericardiocentesis was not emergently performed. The findings of tamponade using point-of-care ultrasound seen in our patient included a greater than 30% reduction of flow velocity with inspiration on pulse wave Doppler assessments of mitral flow. A distended inferior vena cava is a non-specific finding in tamponade. The most specific finding is a diastolic collapse of the right ventricle.6 The utility of bedside pericardiocentesis in unstable patients is debatable as it can aggravate the risk of leak and complicate surgical repair.6 However, removal of even a small amount of blood can help maintain a systolic blood pressure of approximately 90 mmHg.7
AD can also lead to coronary malperfusion, which can mimic several other conditions.8 In cases of dissection affecting the aortic root, coronary artery malperfusion can present as ST-segment elevation or non-ST-segment elevation myocardial infarction. As a result, approximately one-quarter of all patients presenting with an AD will have a positive troponin, as our patient did.9 Additionally, up to 8% of patients with type A dissection have acute ischemic changes.10 In our patient, the dissection extended into the left and right coronary arteries, but the ECG lacked any ischemic findings.
Patients with AD are at risk of rapid deterioration and initial management should include strict control of blood pressure and heart rate, given the shearing forces in the aortic wall and pain control. However, definitive treatment is largely based on the location of the dissection. Most guidelines describe therapeutic goals including a heart rate of lower than 60 beats per minute and systolic blood pressure less than 120 mmHg.11 Beta-blockers are the preferred pharmacologic agents as they can concomitantly lower the heart rate and blood pressure. Intravenous esmolol or labetalol are the preferred single agents. Emergent surgery is usually performed for type A dissection and frequently for type B if medical management fails.
Conclusion
AD is a life-threatening disease that accounts for a small fraction of the causes of chest pain that presents to the ED. CT angiography is the common imaging method for diagnosis; however, TTE and transesophageal echocardiography may provide additional information or help diagnose cases in the absence of CT scan. Treatment is focused on strict management of blood pressure, heart rate, and pain, as well as prompt surgical repair.
Disclosure
The authors declared no conflicts of interest. Written consent was obtained from the patient.
references
- 1. Ramanath VS, Oh JK, Sundt III TM, Eagle KA. Acute aortic syndromes and thoracic aortic aneurysm. Mayo Clinic Proceedings 2009 May;84(5):465-481.
- 2. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al.; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American College of Radiology; American Stroke Association; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of Thoracic Surgeons; Society for Vascular Medicine. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. J Am Coll Cardiol 2010 Apr;55(14):e27-e129.
- 3. Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U, et al; IRAD Investigators. Insights from the international registry of acute aortic dissection: a 20-year experience of collaborative clinical research. Circulation 2018 Apr;137(17):1846-1860.
- 4. Upadhye S, Schiff K. Acute aortic dissection in the emergency department: diagnostic challenges and evidence-based management. Emerg Med Clin North Am 2012 May;30(2):307-327.
- 5. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA 2000 Feb;283(7):897-903.
- 6. Cruz I, Stuart B, Caldeira D, Morgado G, Gomes AC, Almeida AR, et al. Controlled pericardiocentesis in patients with cardiac tamponade complicating aortic dissection: experience of a centre without cardiothoracic surgery. Eur Heart J Acute Cardiovasc Care 2015 Apr;4(2):124-128.
- 7. Klein AL, Abbara S, Agler DA, Appleton CP, Asher CR, Hoit B, et al. American society of echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the society for cardiovascular magnetic resonance and society of cardiovascular computed tomography. J Am Soc Echocardiogr 2013 Sep;26(9):965-1012.e15.
- 8. Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, et al; ESC Scientific Document Group. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC) endorsed by: the European association for cardio-thoracic surgery (EACTS). Eur Heart J 2015 Nov;36(42):2921-2964.
- 9. Cambria RP, Brewster DC, Gertler J, Moncure AC, Gusberg R, Tilson MD, et al. Vascular complications associated with spontaneous aortic dissection. J Vasc Surg 1988 Feb;7(2):199-209.
- 10. Vrsalovic M. Prognostic effect of cardiac troponin elevation in acute aortic dissection: a meta-analysis. Int J Cardiol 2016 Jul;214:277-278.
- 11. Hirata K, Wake M, Takahashi T, Nakazato J, Yagi N, Miyagi T, et al. Clinical predictors for delayed or inappropriate initial diagnosis of type A acute aortic dissection in the emergency room. PLoS One 2015 Nov;10(11):e0141929.