Neonatal sepsis imposes a substantial burden on the healthcare systems of lower-and-middle-income countries (LMICs). Reports have previously estimated a global mortality rate of 17.6% for early-onset sepsis (EOS) and 16.4% for late-onset sepsis (LOS).1 In LMICs, neonates with clinically suspected sepsis or those with laboratory-supported findings face significantly increased all-cause mortality risks by five- and nine-fold, respectively.2 Evidence from Vietnam has shown that several clinical and laboratory factors are associated with higher risks of neonatal sepsis mortality, including extremely low birth weight, hyperglycemia, thrombocytopenia, leukopenia, sclerema, base excess < -20 mEq/L, and serum lactate > 4 mmol/L.3 Elevated neutrophil-lymphocyte-ratio has also been reported as an independent prognostic factor of neonatal sepsis mortality (hazard ratio (HR) = 7.52; p = 0.001), with an 80% sensitivity and 65.8% specificity for the cutoff value of 7.65.4,5 In a resource-limited setting, the use of these predictors is crucial for determining appropriate management and prognosis of neonatal sepsis patients.
Blood culture is the gold standard diagnostic procedure for neonates with clinically suspected neonatal sepsis.6 Since the introduction of continuous blood culture monitoring devices over 30 years ago, the term time to positivity (TTP) has been commonly used by clinicians to indicate the time lapsed from initial culture incubation to a report of positive growth signal on the instrument.7–9 Generally, TTP provides an indirect representation of bacterial load and/or rate of virulence of a certain bloodstream infection, implying that causative pathogens with higher growth rates will be detected earlier rate in the procedure.9–11 A systematic review and meta-analysis suggested that a shorter TTP serves as a crucial predictor of overall survival and septic shock in bloodstream infections.12 TTP also plays an important role in determining the duration of empirical antibiotics administration following the clinical diagnosis of sepsis and blood specimen collection.13 Both microbiological and clinical characteristics were recognized as determinant factors of TTP, but the interpretation of their association differs: from a microbiological standpoint, a faster or slower TTP indicates the inherent growth capacity of the organism, whereas clinically, it reflects differences in the inoculum originally present in the blood.14
However, it is crucial to highlight that TTP is also heavily associated with commonly overlooked confounding factors found in clinical practice, primarily those related to logistics (transportation, administration, opening hours, etc.).9 Although the evaluation of TTP in neonatal sepsis has been thoroughly explored in numerous reports, the lack of evidence originating from LMICs and the aforementioned reasons may potentially reveal notable discrepancies of results.15–19 In this study, we aimed to observe the TTP of neonatal sepsis cases from a tertiary healthcare center in Indonesia, as well as analyze its differences among different causative pathogens and its prognostic capability to predict overall survival.
Methods
A retrospective cohort study was conducted at Dr. Soetomo General Hospital, a referral healthcare center for neonatal sepsis in the Eastern Region of Indonesia. All neonates with bacteriological confirmation of neonatal sepsis from the neonatal intensive care unit (NICU) from 1 January 2020 to 31 August 2022, were included in this study. The identification of neonatal sepsis cases was based on the electronic medical records under the International Classification of Diseases-10 (ICD-10) code P36.
Retrospective identification was conducted on all initial blood cultures collected after birth, in which organisms were isolated and subsequently confirmed as the causative pathogens of neonatal sepsis. Blood specimens for culture were collected with a minimum volume of 1 mL before any administration of empirical antibiotics. The microbiological culture procedure was conducted at the Clinical Microbiology Laboratory of Dr. Soetomo General Hospital using the automated and continuous detection systems of BD BACTEC. For neonates who underwent multiple blood culture procedures, only the initial blood culture was analyzed for this study.
Information regarding clinical characteristics of neonatal sepsis was extracted from the electronic medical records that fulfilled the following eligibility criteria: 1) neonates were delivered inside the healthcare facility, 2) complete maternal history records, and 3) the first identified case of neonatal sepsis in each patient. The following data were extracted for each neonate: sex, gestational age, birth weight, mode of delivery, sepsis onset, presence of birth asphyxia, neonatal outcome (mortality), and risk factors associated with maternal history (premature rupture of membranes (PROMs), preeclampsia, antenatal corticosteroids, urinary tract infection (UTI), and anemia of pregnancy).
Continuous variables were presented as median with IQR and categorical variables were presented as frequency and percentages. The comparison of neonatal and maternal characteristics with overall survival was conducted categorically using the chi-square test, while the Mann-Whitney test was applied for the continuous comparison of TTP with overall survival. The distribution of causative pathogens was listed along with their respective median (IQR) TTP, as well as the proportion of positive cultures at 24, 48, 72, and 96 hours. Kaplan-Meier curves were generated to plot the proportion of positive cultures over time, compare the TTP between different species, and identify overall survival based on different TTP ranges. The comparison between curves was conducted using the log-rank test. To determine the prognostic capability of TTP and other variables for overall survival, a backward-stepwise Cox multivariate regression model was implemented. Variables were included in the model based on a score statistic of < 0.05, and any variables with a score statistic > 0.25 were excluded. The measures of risk for this model were expressed as a HR with its respective 95% CI. Statistical significance was determined if the p-value was < 0.05. All statistical analyses were conducted with IBM SPSS Statistics for Windows (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) and GraphPad Prism 9.5.1.
Results
During the study period, a total of 569 neonates were clinically diagnosed with neonatal sepsis and received treatments in the NICU of Dr. Soetomo General Hospital. Out of the 569 neonates, 243 were outpatient neonates, 11 neonates with incomplete medical records, and 49 neonates who did not undergo blood culture confirmation were excluded from the analysis. Of the 266 eligible neonates with blood specimens taken for culture, 141 showed no growth in blood cultures or were considered contaminants (53.0%). Thus, 125 neonatal blood cultures and their associated clinical records were included in our analysis [Table 1]. Most of the study cohort consisted of LOS cases (76.8%), male sex (52.8%), gestational age of 32–36 weeks (46.4%), and birth weight of 1500–<2500 (37.6%). Maternal and perinatal histories identified a number of cases of birth asphyxia (12.8%), cesarean delivery (78.4%), PROM (16.0%), preeclampsia (45.6%), antenatal corticosteroid use (51.2%), intrapartum fever (4.8%), maternal UTI (2.4%), and anemia of pregnancy (30.4%). Among the bacteriologically confirmed cases of neonatal sepsis, the TTP of blood cultures ranged from 15.1 to 143.0 h, with a median of 58.1 h. The all-cause mortality rate for the study cohort was 49.6%, with a median length of stay of 22 days.
Table 1: Characteristics of the study cohort
(N = 125).
|
Late-onset sepsis
|
96 (76.8)
|
|
Male
|
66 (52.8)
|
|
Gestational age, median (IQR), weeks
|
33 (5)
|
|
Gestational age, weeks
|
|
|
< 28
|
15 (12.0)
|
|
28–31
|
35 (28.0)
|
|
32–36
|
58 (46.4)
|
|
≥ 37
|
17 (13.6)
|
|
Birth weight, median (IQR), g
|
1450 (1000)
|
|
Birth weight, g
|
|
|
< 1000
|
24 (19.2)
|
|
1000–< 1500
|
39 (31.2)
|
|
1500–< 2500
|
47 (37.6)
|
|
≥ 2500
|
15 (12.0)
|
|
Birth asphyxia
|
16 (12.8)
|
|
Cesarean delivery
|
98 (78.4)
|
|
Maternal history
|
|
|
PROM
|
20 (16.0)
|
|
Preeclampsia
|
57 (45.6)
|
|
Antenatal corticosteroid
|
64 (51.2)
|
|
Intrapartum fever
|
6 (4.8)
|
|
Maternal UTI
|
3 (2.4)
|
|
Anemia of pregnancy
|
38 (30.4)
|
|
Time to positivity, median (IQR), h
|
58.08 (24.48)
|
|
Time to positivity, h
|
|
|
< 48
|
50 (40.0)
|
|
48–72
|
55 (44.0)
|
|
> 72
|
20 (16.0)
|
|
Length of stay, median (IQR), days
|
22 (26)
|
PROM: premature rupture of membranes, UTI: urinary tract infection.
Among the causative pathogens, gram-negative bacteria accounted for the majority (62.4%) of pathogen growth from blood cultures [Table 2]. The most frequently isolated gram-negative organisms were Klebsiella pneumoniae (47.4%), Acinetobacter baumannii (20.5%), and Enterobacter cloacae (14.1%). No fungal species were isolated from the blood cultures. In the entire sample, 41.6% of blood cultures were positive by 48 hours, 86.4% by 72 hours, and 98.4% by 96 hours [Figure 1a]. Gram-negative bacteria showed a relatively lower median TTP compared to gram-positive bacteria (47.76 vs. 66.24 hours). The shortest TTP was identified in a blood culture showing the growth of Lelliottia amnigena biogroup 1 at 15.12 h. Conversely, blood cultures yielding Pseudomonas spp. had the longest median TTP at 95.28 h. There were four neonatal blood cultures with more than one pathogen grown and isolated including combinations of 1) Staphylococcus sciuri (CoNS) and Stenotrophomonas maltophila; 2) Serratia plymuthica and E. cloacae; 3) K. pneumoniae and Escherichia coli; as well as 4) A. baumannii and S. aureus. These polybacterial cultures had a median TTP of 59.52 h placing them between the median TTP of gram-negative and gram-positive bacteria. Marked differences in TTP were identified among the four major causative pathogens of neonatal species (p = 0.010) [Figure 1b]. However, these differences were only found in four subgroup comparisons between K. pneumoniae and CoNS (p = 0.027), A. baumannii and CoNS (p = 0.011), E. cloacae and CoNS (p < 0.001), and E. cloacae and A. baumannii (p = 0.039) [Figure 2].
Table 2: Time to positivity (TTP) by species (N = 125).
|
Gram-negative
|
78 (62.4)
|
47.76 (22.56)
|
2.6
|
50.0
|
89.7
|
97.4
|
|
Klebsiella pneumoniae
|
37 (47.4)
|
48.00 (22.32)
|
0.0
|
48.9
|
89.2
|
97.3
|
|
Acinetobacter baumannii
|
16 (20.5)
|
58.32 (23.28)
|
6.3
|
43.8
|
93.8
|
100
|
|
Enterobacter cloacae
|
11 (14.1)
|
46.32 (16.32)
|
0.0
|
63.6
|
100
|
100
|
|
Escherichia coli
|
5 (6.4)
|
53.28 (23.76)
|
0.0
|
40.0
|
80.0
|
100
|
|
Klebsiella ozaenae
|
2 (2.6)
|
45.12 (0.72)
|
0.0
|
100
|
100
|
100
|
|
Pseudomonas spp.
|
2 (2.6)
|
95.28 (13.68)
|
0.0
|
0.0
|
0.0
|
50.0
|
|
Achromobacter spp.
|
1 (1.3)
|
42.48
|
0.0
|
100
|
100
|
100
|
|
Aeromonas caviae
|
1 (1.3)
|
29.52
|
0.0
|
100
|
100
|
100
|
|
Serratia marcescens
|
1 (1.3)
|
52.80
|
0.0
|
0.0
|
100
|
100
|
|
Serratia plymuthica
|
1 (1.3)
|
57.36
|
0.0
|
0.0
|
100
|
100
|
|
Lelliottia amnigena
|
1 (1.3)
|
15.12
|
100
|
100
|
100
|
100
|
|
Gram-positive
|
43 (34.4)
|
66.24 (13.92)
|
0.0
|
23.3
|
74.4
|
100
|
|
Staphylococcus sciuri
|
36 (83.7)
|
66.72 (12.96)
|
0.0
|
22.2
|
75.0
|
100
|
|
Staphylococcus aureus
|
2 (4.7)
|
60.96 (1.44)
|
0.0
|
0.0
|
100
|
100
|
|
Bacillus spp.
|
2 (4.7)
|
54.96 (18.48)
|
0.0
|
50.0
|
50.0
|
100
|
|
Streptococcus agalactiae
|
1 (2.3)
|
30.00
|
0.0
|
100
|
100
|
100
|
|
Enterococcus faecalis
|
1 (2.3)
|
65.04
|
0.0
|
0.0
|
100
|
100
|
|
Corynebacterium
|
1 (2.3)
|
93.60
|
0.0
|
0.0
|
0.0
|
100
|
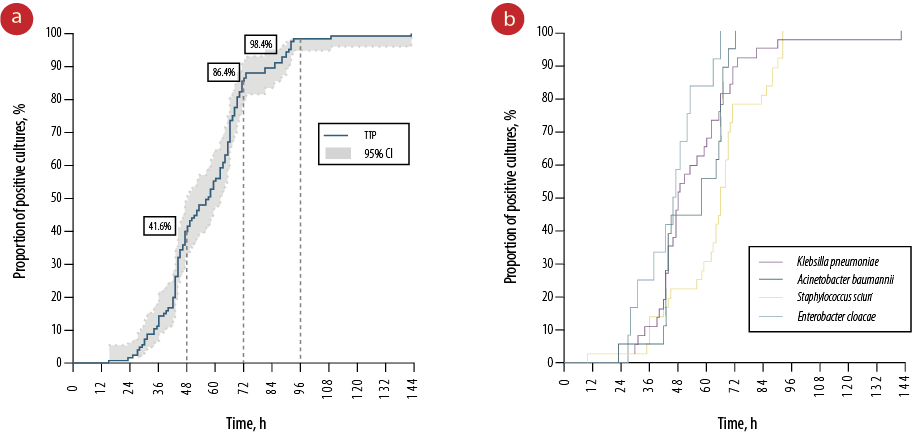 Figure 1: (a) Kaplan-Meier curve depicting the proportion of positive blood cultures by time. (b) Kaplan-Meier curves illustrating the positive blood culture rates by time across various species (p = 0.010).
Figure 1: (a) Kaplan-Meier curve depicting the proportion of positive blood cultures by time. (b) Kaplan-Meier curves illustrating the positive blood culture rates by time across various species (p = 0.010).
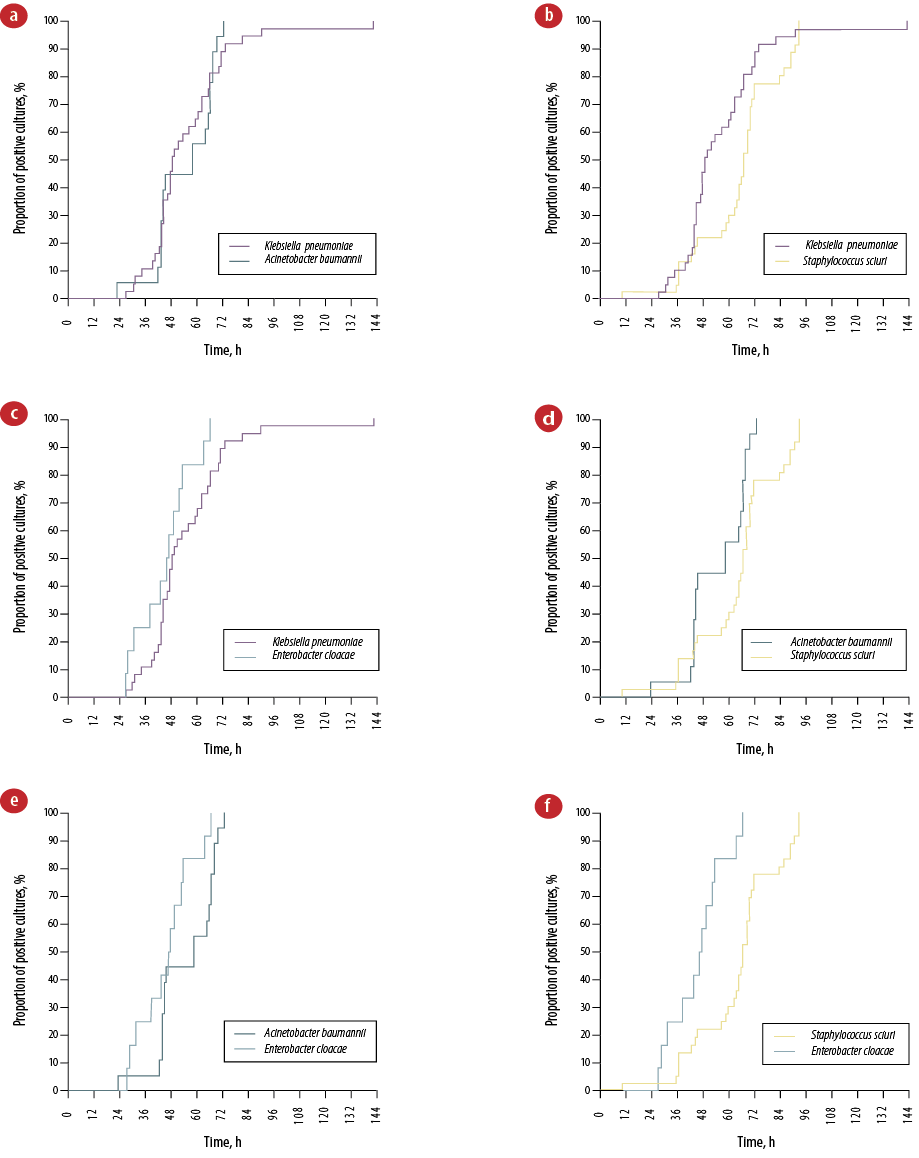 Figure 2: Kaplan-Meier curves representing the time to positivity by species subgroups: (a) Klebsiella pneumoniae and Acinetobacter baumannii (p = 0.812),(b) K. pneumoniae and Staphylococcus sciuri (CoNS)
Figure 2: Kaplan-Meier curves representing the time to positivity by species subgroups: (a) Klebsiella pneumoniae and Acinetobacter baumannii (p = 0.812),(b) K. pneumoniae and Staphylococcus sciuri (CoNS)
(p = 0.027), (c) K.pneumoniae and Enterobacter cloacae (p = 0.096), (d) A. baumannii and CoNS (p = 0.011), (e) A. baumannii and E. cloacae (p = 0.039), and (f) CoNS and E. cloacae (p < 0.001).
Significant differences were observed between gestational age (p = 0.020), birth weight (p = 0.026), birth asphyxia (p = 0.007), antenatal corticosteroid use (p = 0.040), and length of stay (p < 0.001) in relation to overall survival within the entire study cohort [Table 3]. Neonates with monobacterial gram-negative sepsis exhibited a significantly lower rate of survivability (p < 0.001), while 100% mortality was evident in all four cases of polybacterial culture. Although no significant differences were identified in the Mann-Whitney test, notable significant differences in the Kaplan-Meier curve were identified among the three categorical groupings of TTP (< 48 h, 48–72 h, > 72 h; p = 0.036) [Figure 3]. Two Cox multivariable regression models were constructed: one for the entire cohort and one for neonates with gram-negative sepsis [Table 4]. The model for the entire cohort encompassed five variables, including sepsis onset, sex, gestational age, birth asphyxia, and TTP. LOS and longer TTP emerged as independent predictors for higher survivability (HR = 0.423, 95% CI: 0.229–0.781; p = 0.006 and HR = 0.985, 95% CI: 0.973–0.998; p = 0.027, respectively). Conversely, the presence of birth asphyxia increases the hazard of mortality by a factor of four among patients with bacteriologically confirmed neonatal sepsis (HR = 4.095, 95% CI: 2.095–8.005; p < 0.001). Within the gram-negative sepsis model, four variables were integrated: sepsis onset, gestational age, birth asphyxia, and TTP. Gestational age < 28 weeks and birth asphyxia were associated with mortality in gram-negative neonatal sepsis (HR = 3.472, 95% CI: 1.065–11.319; p = 0.039 and HR = 6.662, 95% CI: 2.495–17.784; p < 0.001, respectively), while LOS was independently predictive of overall survival (HR = 0.356, 95% CI: 0.171–0.742; p = 0.006). Additionally, a longer TTP in gram-negative neonatal sepsis was also marginally predictive of overall survival, with a 1.7% increase in odds for every one-hour increase in TTP (HR = 0.983, 95% CI: 0.968–0.999; p = 0.033).
Table 3: Comparison of factors with overall survival (N = 125).
|
Onset
|
|
Early
|
10 (34.5)
|
19 (65.5)
|
2.342 (0.986–5.562)
|
0.050
|
|
Late
|
53 (55.2)
|
43 (44.8)
|
ref.
|
|
Sex
|
|
Male
|
36 (54.5)
|
30 (45.5)
|
0.703 (0.347–1.423)
|
0.327
|
|
Female
|
27 (45.8)
|
32 (54.2)
|
ref.
|
|
Gestational age, weeks
|
|
< 28
|
2 (13.3)
|
13 (86.7)
|
7.312 (1.249–42.813)
|
0.020*
|
|
28–31
|
21 (60.0)
|
14 (40.0)
|
0.750 (0.233–2.412)
|
|
32–36
|
31 (53.4)
|
27 (46.6)
|
0.980 (0.332–2.894)
|
|
≥ 37
|
9 (52.9)
|
8 (47.1)
|
ref.
|
|
Birth weight, g
|
|
< 1000
|
7 (29.2)
|
17 (70.8)
|
2.125 (0.555–8.140)
|
0.026*
|
|
1000–< 1500
|
18 (46.2)
|
21 (53.8)
|
1.021 (0.309–3.369)
|
|
1500–< 2500
|
31 (66.0)
|
16 (34.0)
|
0.452 (0.139–1.470)
|
|
≥ 2500
|
7 (46.7)
|
8 (53.3)
|
ref.
|
|
Birth asphyxia
|
|
Yes
|
3 (18.8)
|
13 (81.3)
|
5.306 (1.430–19.683)
|
0.007*
|
|
No
|
60 (55.0)
|
49 (45.0)
|
ref.
|
|
Mode of delivery
|
|
Vaginal
|
11 (40.7)
|
16 (59.3)
|
1.644 (0.693–3.902)
|
0.257
|
|
Cesarean
|
52 (53.1)
|
46 (46.9)
|
ref.
|
|
PROM
|
|
Yes
|
9 (45.0)
|
11 (55.0)
|
1.294 (0.495–3.381)
|
0.598
|
|
No
|
53 (51.4)
|
51 (49.0)
|
ref.
|
|
Preeclampsia
|
|
Yes
|
27 (47.4)
|
30 (52.0)
|
1.250 (0.618–2.530)
|
0.535
|
|
No
|
36 (52.9)
|
32 (47.1)
|
ref.
|
|
Antenatal corticosteroid use
|
|
Yes
|
38 (59.4)
|
26 (40.6)
|
0.475 (0.233–0.970)
|
0.040*
|
|
No
|
25 (41.0)
|
36 (59.0)
|
ref.
|
|
Intrapartum fever
|
|
Yes
|
3 (50.0)
|
3 (50.0)
|
1.017 (0.197–5.243)
|
1.000
|
|
No
|
60 (50.4)
|
59 (49.6)
|
ref.
|
|
Maternal UTI
|
|
Yes
|
0 (0)
|
3 (100)
|
N/A
|
0.119
|
|
No
|
63 (51.6)
|
59 (48.4)
|
|
Anemia of pregnancy
|
|
Yes
|
20 (52.6)
|
18 (47.4)
|
0.880 (0.410–1.886)
|
0.742
|
|
No
|
43 (49.4)
|
44 (50.6)
|
ref.
|
|
Gram staining
|
|
Monobacterial gram-positive
|
32 (74.4)
|
11 (25.6)
|
ref.
|
< 0.001*
|
|
Monobacterial gram-negative
|
31 (39.7)
|
47 (60.3)
|
4.411 (1.940–10.030)
|
|
Polybacterial
|
0 (0)
|
4 (100)
|
N/A
|
|
TTP, median (IQR)#, h
|
62.64 (28.08)
|
50.28 (21.66)
|
N/A
|
0.137
|
|
TTP, h
|
|
< 48
|
23 (46.0)
|
27 (54.0)
|
3.522 (1.110–11.176)
|
0.056
|
|
48–72
|
25 (45.5)
|
30 (54.5)
|
3.600 (1.148–11.288)
|
|
> 72
|
15 (75.0)
|
5 (25.0)
|
ref.
|
*Statistical significance, p < 0.05. #Mann-Whitney test. RR: relative risk; ref: reference category; PROM: premature rupture of membranes; UTI: urinary tract infection; TTP: time to positivity; N/A: not applicable.
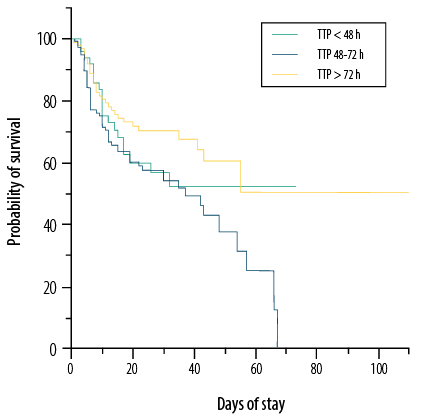 Figure 3: Kaplan-Meier curves of overall survival based on different range of time to positivity (TTP) (p = 0.036).
Figure 3: Kaplan-Meier curves of overall survival based on different range of time to positivity (TTP) (p = 0.036).
Table 4: Cox proportional hazard regression model of overall survival.
|
Sex
|
|
|
|
|
|
|
|
Male
|
36 (54.5)
|
ref.
|
ref.
|
|
|
|
|
Female
|
27 (45.8)
|
1.612 (0.904–2.875)
|
0.105
|
|
|
|
|
Onset
|
|
|
|
|
|
|
|
Early
|
19 (65.5)
|
ref.
|
ref.
|
14 (70.0)
|
ref.
|
ref.
|
|
Late
|
43 (44.8)
|
0.423 (0.229–0.781)
|
0.006*
|
33 (56.9)
|
0.356 (0.171–0.742)
|
0.006*
|
|
Gestational age, weeks
|
|
< 28
|
13 (86.7)
|
1.893 (0.737–4.865)
|
0.185
|
9 (90.0)
|
3.472 (1.065–11.319)
|
0.039*
|
|
28–32
|
14 (40.0)
|
0.704 (0.290–1.711)
|
0.438
|
11 (52.4)
|
0.793 (0.264–2.386)
|
0.680
|
|
32–36
|
27 (46.6)
|
1.317 (0.583–2.978)
|
0.508
|
22 (61.1)
|
1.769 (0.648–4.832)
|
0.266
|
|
≥ 37
|
8 (47.1)
|
ref.
|
ref.
|
6 (54.5)
|
ref.
|
ref.
|
|
Birth asphyxia
|
13 (81.3)
|
4.095 (2.095–8.005)
|
< 0.0001*
|
7 (87.5)
|
6.662 (2.495–17.784)
|
< 0.001*
|
*Statistical significance, p < 0.05. #Data presented as median (IQR). HR: hazard ratio; ref: reference category.
Discussion
In comparison to previous studies, our findings revealed a higher median TTP of 58.1 h, with the majority of positive cultures were identified between 48 and 72 hours. For instance, Abdelhamid et al,19 reported a median TTP of 21.1 h for pathogenic organisms causing neonatal sepsis. Another study on EOS demonstrated that 68% of positive blood cultures were identified within 24 hours, 94% within 36 hours, and 97% within 48 hours.20 It is important to note that our study considered the time when blood specimens enter the laboratory for processing (inoculation and incubation), providing a more clinically applicable interpretation of results. This approach differs from most studies that define TTP from the start of incubation, and it addresses potential influences of technical factors such as transportation and laboratory logistics. An illustrative instance of this line of reasoning is evidenced in a study carried out by Cobos-Trigueros et al,21 which reported significant differences in TTP for Candida glabrata between centers in Barcelona, Spain and Cologne, Germany (80.8 h vs. 53.4 h, respectively); presumably as a result of the unequal distribution of cases and loading time between the centers. This further underscores the impact of the geographical context in which the study was undertaken, given the inherent constraints imposed by the limited availability of laboratory facilities and human resources in LMICs.22,23 Furthermore, our study indicates that recommendations on empirical antibiotic withdrawal after 24 or 36 hours, as presented in previous reports, may not be applicable in resource-limited settings.15,16,18,20 The reasons underlying this practice are inherently complex, as healthcare providers may need to administer empirical antibiotics until the blood culture results are available for review.24,25 It is also crucial to emphasize that empirical antibiotic administration has been shown to elongate the length of stay in the NICU,26,27 and alter the normal body microbiome during a critical developmental period for an infant.28,29 Hence, the measurement of TTP can provide valuable information for the antibiotic stewardship team to consider in developing an antibiotic de-escalation strategy.
Variations in TTP among pathogens causing neonatal sepsis were observed. All gram-negatives (K. pneumoniae, A. baumannii, and E. cloacae) exhibited significantly shorter TTP compared to the most common gram-positives pathogen (CoNS). A previous study conducted on patients with neutropenia highlighted similar differences between both groups of pathogens, showing that monomicrobial gram-positive and CoNS bacteremia had 2.47 and 3.92 times higher risks of yielding TTP longer than 24 hours, respectively.30 Furthermore, the study demonstrated that all monomicrobial gram-negative aerobic bacteremia cases had a TTP of < 24 h, with 48% higher odds of yielding positivity within 16 hours.30 Another study demonstrated that the median TTP of suspected EOS and LOS due to monobacterial gram-positive organisms was longer than those caused by monobacterial gram-negative organisms (23.1 h vs. 17.0 h).19 However, it may be inappropriate to generalize the differences solely based on Gram staining, as the variations could be attributed to the level of specific species. An earlier study exploring this approach showed that even among gram-negative bacilli, a significantly shorter TTP was identified between infections caused by E. coli, Klebsiella spp., Enterobacter spp., Citrobacter spp., and Aeromonas spp. Compared to those caused by Proteeae, Salmonella spp., Serratia spp., P. aeruginosa, and other non-fermenters.14 In our study, a significant difference in TTP among gram-negative isolates was only identified on one comparison between E. cloacae and A. baumannii (p = 0.039). Nevertheless, it should also be emphasized that determining CoNS as causative gram-positive pathogens required extensive consideration as it is commonly associated with contaminants; and therefore, were excluded in the analysis of TTP in prior studies.16–18,31 A study conducted on 0 to 90-day infants from an emergency department showed a significantly shorter TTP of pathogenic organisms compared to contaminants and stated that an incubation period of 36 h was adequate to detect 100% blood culture for significant pathogens.32 Additionally, Huggard et al,16 (2021) identified that shorter TTP is associated with gram-negative neonatal sepsis and a 15.5% increase in the odds of isolating a pathogenic organism compared to contaminants (including CoNS). Further adjustment on both patient-specific and culture-specific factors also suggested that the odds of obtaining TTP > 36 h were 14 times higher for CoNS (aodds ratio = 14.60, 95% CI: 6.98–30.58; p < 0.001).18
This study is the first study to evaluate the prognostic potential of blood culture TTP for mortality in culture-proven neonatal sepsis. We found that a longer TTP marginally predicts better prognosis for overall survival in neonates with sepsis. Mortality risk decreased by 1.5% and 1.7% for every hour increase in TTP in the entire cohort and gram-negative sepsis cases, respectively. Comparable studies have linked shorter TTP with a better prognosis. For instance, a study in Ireland showed lower neonatal sepsis mortality rates when TTP consistently remained under 24 hours.16 A previous report conducted on children with Pseudomonas aeruginosa bacteremia also showed the prognostic role of TTP ≤ 18 h to predict in-hospital mortality (odds ratio = 5.88, 95% CI: 1.21–21.96; p = 0.035).33 In gram-positive neonatal sepsis, TTP ≤ 12 and ≤ 17 h have also been previously associated with in-hospital mortality among children with Streptococcus pneumoniae and S. aureus bacteremia, respectively.34,35
Additionally, our study also found that EOS, extremely preterm birth (gestational age < 28 weeks), and birth asphyxia were associated with worse outcomes in neonatal sepsis cases. It is noteworthy to emphasize that both prematurity and intrapartum complications (such as birth asphyxia) are the two most common causes of neonatal death worldwide; thus, rendering the outcome not necessarily surprising.36,37 A systematic review also suggested that neonatal mortality was estimated at a higher rate for EOS compared to LOS, presumably attributed to a higher incidence of other risk factors in those groups (prematurity and low birth weight).1 Previous studies have highlighted that maternal history of pre-eclampsia/eclampsia and antepartum hemorrhage were significantly associated with mortality in neonatal sepsis.38 Higher mortality was also associated with a maternal history of abortions, abnormal placenta, and PROM.39 However, the current study did not find any significant association between maternal history and neonatal sepsis mortality. It is important to note that invasive procedures such as mechanical ventilation and total parenteral nutrition, which have been shown to increase the risk of poor outcomes in neonatal sepsis,40 were not accounted for in this study due to the insufficient information available in the medical records.
Though insightful, this study had limitations. The sample size was relatively small and a longer cohort period would have enhanced robustness. Data gaps in perinatal antibiotic use, empirical antibiotic types, and therapeutic modalities were present. Moreover, the timing of bottle loading following blood specimen collection was unaccounted for. Finally, survival analysis was conducted only for the entire cohort and gram-negative sepsis cases, not specific pathogen-caused sepsis.
Conclusion
TTP predicts neonatal sepsis pathogen species and overall survival. The study highlighted K. pneumoniae, A. baumannii, and E. cloacae as the three most common gram-negative pathogens causing neonatal sepsis and exhibited significantly shorter TTP compared to the most common gram-positive pathogens, CoNS. E. cloacae also showed a significantly shorter TTP compared to A. baumannii. A shorter TTP, EOS, and birth asphyxia were independent prognostic factors for in-hospital mortality for the entire cohort and the gram-negative sepsis cohort. Additionally, extremely preterm birth was also associated with mortality in gram-negative neonatal sepsis.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
References
- 1. Fleischmann C, Reichert F, Cassini A, Horner R, Harder T, Markwart R, et al. Global incidence and mortality of neonatal sepsis: a systematic review and meta-analysis. Arch Dis Child 2021 Jan;106(8):745-752.
- 2. Milton R, Gillespie D, Dyer C, Taiyari K, Carvalho MJ, Thomson K, et al; BARNARDS Group. Neonatal sepsis and mortality in low-income and middle-income countries from a facility-based birth cohort: an international multisite prospective observational study. Lancet Glob Health 2022 May;10(5):e661-e672.
- 3. Toan ND, Darton TC, Huong NH, Nhat LT, Nguyen TN, Tuyen HT, et al. Clinical and laboratory factors associated with neonatal sepsis mortality at a major Vietnamese children’s hospital. PLOS Glob Public Health 2022 Sep;2(9):e0000875.
- 4. Xia X, Wang Y, Xie M, Qiu S, Zhou J. Elevated neutrophil - to - monocyte ratio as a prognostic marker for poor outcomes in neonatal sepsis. Heliyon 2022 Oct;8(10):e11181.
- 5. Sumitro KR, Utomo MT, Widodo AD. Neutrophil-to-lymphocyte ratio as an alternative marker of neonatal sepsis in developing countries. Oman Med J 2021 Jan;36(1):e214.
- 6. Puopolo KM, Benitz WE, Zaoutis TE; COMMITTEE ON FETUS AND NEWBORN; COMMITTEE ON INFECTIOUS DISEASES. Management of neonates born at ≥35 0/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics 2018 Dec;142(6):e20182894.
- 7. Rogers MS, Oppenheim BA. The use of continuous monitoring blood culture systems in the diagnosis of catheter related sepsis. J Clin Pathol 1998 Aug;51(8):635-637.
- 8. Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis 1997 Apr;24(4):584-602.
- 9. Lamy B. Blood culture time-to-positivity: making use of the hidden information. Clin Microbiol Infect 2019 Mar;25(3):268-271.
- 10. George BJ, Horvath LL, Hospenthal DR. Effect of inoculum size on detection of Candida growth by the BACTEC 9240 automated blood culture system using aerobic and anaerobic media. J Clin Microbiol 2005 Jan;43(1):433-435.
- 11. Haimi-Cohen Y, Vellozzi EM, Rubin LG. Initial concentration of Staphylococcus epidermidis in simulated pediatric blood cultures correlates with time to positive results with the automated, continuously monitored BACTEC blood culture system. J Clin Microbiol 2002 Mar;40(3):898-901.
- 12. Hsieh YC, Chen HL, Lin SY, Chen TC, Lu PL. Short time to positivity of blood culture predicts mortality and septic shock in bacteremic patients: a systematic review and meta-analysis. BMC Infect Dis 2022 Feb;22(1):142.
- 13. Lambregts MM, Bernards AT, van der Beek MT, Visser LG, de Boer MG. Time to positivity of blood cultures supports early re-evaluation of empiric broad-spectrum antimicrobial therapy. PLoS One 2019 Jan;14(1):e0208819.
- 14. Martínez JA, Pozo L, Almela M, Marco F, Soriano A, López F, et al. Microbial and clinical determinants of time-to-positivity in patients with bacteraemia. Clin Microbiol Infect 2007 Jul;13(7):709-716.
- 15. Arias-Felipe A, Ramírez-Berrios J, Recio-Martinez R, Orellana-Miguel MA, Fontiveros-Escalona D, Bergón-Sendín E, et al. Determining time to positivity of blood cultures in a neonatal unit. J Pediatric Infect Dis Soc 2022 Dec;11(11):510-513.
- 16. Huggard D, Powell J, Kirkham C, Power L, O’Connell NH, Philip RK. Time to positivity (TTP) of neonatal blood cultures: a trend analysis over a decade from Ireland. J Matern Fetal Neonatal Med 2021 Mar;34(5):780-786.
- 17. Coggins SA, Harris MC, Srinivasan L. Dual-site blood culture yield and time to positivity in neonatal late-onset sepsis. Arch Dis Child Fetal Neonatal Ed 2022 Sep;107(5):475-480.
- 18. Mukhopadhyay S, Briker SM, Flannery DD, Dhudasia MB, Coggins SA, Woodford E, et al. Time to positivity of blood cultures in neonatal late-onset bacteraemia. Arch Dis Child Fetal Neonatal Ed 2022 Nov;107(6):583-588.
- 19. Abdelhamid SM. Time to positivity and antibiotic sensitivity of neonatal blood cultures. J Glob Infect Dis 2017;9(3):102-107.
- 20. Kuzniewicz MW, Mukhopadhyay S, Li S, Walsh EM, Puopolo KM. Time to positivity of neonatal blood cultures for early-onset sepsis. Pediatr Infect Dis J 2020 Jul;39(7):634-640.
- 21. Cobos-Trigueros N, Kaasch AJ, Soriano A, Torres JL, Vergara A, Morata L, et al. Time to positivity and detection of growth in anaerobic blood culture vials predict the presence of Candida glabrata in candidemia: a two-center European cohort study. J Clin Microbiol 2014 Aug;52(8):3082-3084.
- 22. Ombelet S, Barbé B, Affolabi D, Ronat JB, Lompo P, Lunguya O, et al. Best practices of blood cultures in low- and middle-income countries. Front Med (Lausanne) 2019 Jun;6:131.
- 23. Isaac EW, Jalo I, Difa AJ, Poksireni MR, Christianah O, Charanci MS, et al. Bacterial blood isolates in children: conventional vs. bactec automated blood culture system in a tertiary health centre in Gombe, North East Nigeria. Open J Med Microbiol 2022;12(3):101-116.
- 24. Graus JM, Herbozo C, Hernandez R, Pantoja AF, Zegarra J. Managing antibiotics wisely in a neonatal intensive care unit in a low resource setting. J Perinatol 2022 Jul;42(7):965-970.
- 25. Schulman J, Dimand RJ, Lee HC, Duenas GV, Bennett MV, Gould JB. Neonatal intensive care unit antibiotic use. Pediatrics 2015 May;135(5):826-833.
- 26. Kopsidas I, Tsopela GC, Molocha NM, Bouza E, Chorafa E, Chorianopoulou E, et al. Reducing duration of antibiotic use for presumed neonatal early-onset sepsis in Greek NICUs. A “low-hanging fruit” approach. Antibiotics 2021 Mar 9;10(3):275.
- 27. Sourour W, Sanchez V, Sourour M, Burdine J, Lien ER, Nguyen D, et al. The association between prolonged antibiotic use in culture negative infants and length of hospital stay and total hospital costs. Am J Perinatol 2023 Apr;40(5):525-531.
- 28. Neuman H, Forsythe P, Uzan A, Avni O, Koren O. Antibiotics in early life: dysbiosis and the damage done. FEMS Microbiol Rev 2018 Jul;42(4):489-499.
- 29. Vangay P, Ward T, Gerber JS, Knights D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe 2015 May;17(5):553-564.
- 30. Lambregts MM, Warreman EB, Bernards AT, Veelken H, von dem Borne PA, Dekkers OM, et al. Distribution and clinical determinants of time-to-positivity of blood cultures in patients with neutropenia. Eur J Haematol 2018 Feb;100(2):206-214.
- 31. Marchant EA, Boyce GK, Sadarangani M, Lavoie PM. Neonatal sepsis due to coagulase-negative staphylococci. Clin Dev Immunol 2013;2013:586076.
- 32. Lefebvre CE, Renaud C, Chartrand C. Time to positivity of blood cultures in infants 0 to 90 days old presenting to the emergency department: is 36 hours enough? J Pediatric Infect Dis Soc 2017 Mar;6(1):28-32.
- 33. Xu H, Cheng J, Yu Q, Li Q, Yi Q, Luo S, et al. Prognostic role of time to positivity of blood culture in children with Pseudomonas aeruginosa bacteremia. BMC Infect Dis 2020 Sep;20(1):665.
- 34. Li Q, Li Y, Yi Q, Suo F, Tang Y, Luo S, et al. Prognostic roles of time to positivity of blood culture in children with Streptococcus pneumoniae bacteremia. Eur J Clin Microbiol Infect Dis 2019 Mar;38(3):457-465.
- 35. Li Y, Li Q, Zhang G, Ma H, Wu Y, Yi Q, et al. Time to positivity of blood culture is a risk factor for clinical outcomes in Staphylococcus aureus bacteremia children: a retrospective study. BMC Infect Dis 2019 May;19(1):437.
- 36. World Health Organization. Newborn health. Perinatal asphyxia. 2022 [cited 2022 Nov 30]. Available from: https://www.who.int/teams/maternal-newborn-child-adolescent-health-and-ageing/newborn-health/perinatal-asphyxia.
- 37. World Health Organization. Newborns: improving survival and well-being. 2020 [cited 2022 Nov 30]. Available from: https://www.who.int/news-room/fact-sheets/detail/newborns-reducing-mortality.
- 38. Meshram RM, Gajimwar VS, Bhongade SD. Predictors of mortality in outborns with neonatal sepsis: a prospective observational study. Niger Postgrad Med J 2019;26(4):216-222.
- 39. Leal YA, Álvarez-Nemegyei J, Velázquez JR, Rosado-Quiab U, Diego-Rodríguez N, Paz-Baeza E, et al. Risk factors and prognosis for neonatal sepsis in southeastern Mexico: analysis of a four-year historic cohort follow-up. BMC Pregnancy Childbirth 2012 Jun;12:48.
- 40. Turhan EE, Gürsoy T, Ovalı F. Factors which affect mortality in neonatal sepsis. Turk Pediatri Ars 2015 Sep;50(3):170-175.