Placenta accreta spectrum (PAS) is a rare obstetrics emergency that is associated with massive immediate postpartum hemorrhage, which often leads to a hysterectomy. The presence of PAS is thought to be more common due to several factors such as rising maternal age at delivery, multiparity, and the increasing number of cesarean deliveries.1 The current case report demonstrated a rare case of PAS in a normally-situated placenta in a virgin abdomen that was diagnosed intraoperatively. Among those without placenta previa, pregnancy via assisted reproductive technology had been reported as an important predictor, but not cesarean section.2 Yet, PAS in a virgin abdomen is rare but possible.
Case Report
A 41-year-old female, gravida 8 para 7 at 40 weeks two days, presented to our center for severe oligohydramnios with an amniotic fluid index of 2.5 cm. Her antenatal care was uneventful, and she had no history of abdominal or uterine surgery. The patient was then planned for with mechanical induction of labor. Following the procedure, she developed a spontaneous rupture of the membrane with a cervical dilatation of 3 cm and was sent to the labor room for Pitocin augmentation.
In the labor room, the artificial rupture of the membrane noted moderate meconium-stained liquor with continuous cardiotocography showing pathological tracing. Thus, an emergency lower-segment cesarean section was performed under spinal anesthesia. A baby girl was delivered with a thick meconium-stained liquor. Fortunately, she had a good Apgar score. The baby was monitored in special care nursing for meconium aspiration syndrome.
Intraoperatively, there was difficulty to remove the whole bulk of the placenta and no plane was felt in between the placenta and endometrium. There was a presence of indentation at the uterine wall during cord traction [Figure 1]. Therefore, PAS was suspected and the team proceeded with a hysterectomy. She was given a total fluid of five crystalloids and one colloid, and transfused with three pints of packed cells during the procedure. Her mean arterial pressure was maintained at > 65% with good oxygen saturation. The total estimated blood loss was three liters. The uterus was sent for histological examination.
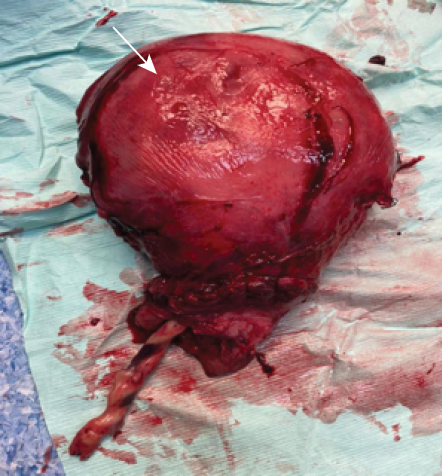 Figure 1: Presence of indentation during separation of the placenta and endometrium layer of the uterus (white arrow).
Figure 1: Presence of indentation during separation of the placenta and endometrium layer of the uterus (white arrow).
Postpartum, she was monitored in a high-dependency suite for one day and was later transferred out to an acute cubicle bed in the postnatal ward. She was then started on venous thromboembolism prophylaxis and discharged when well.
Gross examination of the uterus revealed the placenta adhered to the anterior uterine wall. Histopathological findings were consistent with PA [Figure 2]. Sections from the uterus showed foci areas of chorionic villi (CV) implant directly on the surface of the myometrium without intervening decidua. Fibrin and extravillous trophoblast are often present between the villi and myometrial fibers [Figures 3–5]. The myometrium was not thinned and no perforation was noted.
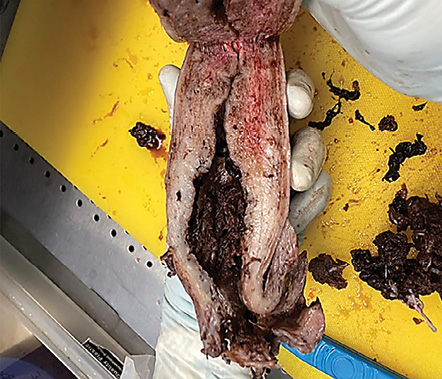 Figure 2: Hemorrhagic uterus with cut section showing the placenta attached to the myometrium of the uterus.
Figure 2: Hemorrhagic uterus with cut section showing the placenta attached to the myometrium of the uterus.
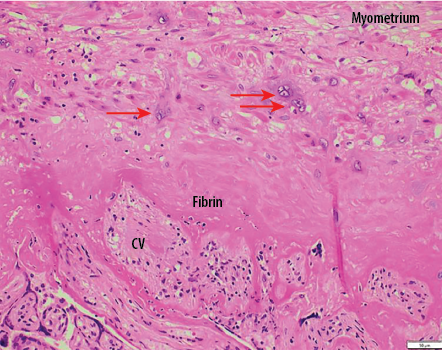 Figure 3: Chorionic villi (CV) implants directly onto myometrial fibers with no intervening decidua. Only extravillous trophoblastic cells (red arrows) and fibrin separate villi from myometrial fibers with no intervening decidua. Hematoxylin and eosin staining, magnification = 200 ×.
Figure 3: Chorionic villi (CV) implants directly onto myometrial fibers with no intervening decidua. Only extravillous trophoblastic cells (red arrows) and fibrin separate villi from myometrial fibers with no intervening decidua. Hematoxylin and eosin staining, magnification = 200 ×.
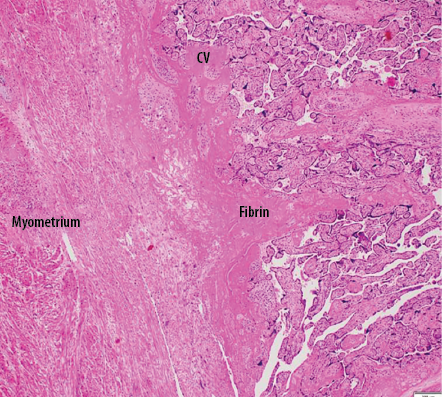 Figure 4: Low power view shows placental villi adhered onto myometrium with no decidual basalis. Hematoxylin and eosin staining, magnification = 40 ×.
Figure 4: Low power view shows placental villi adhered onto myometrium with no decidual basalis. Hematoxylin and eosin staining, magnification = 40 ×.
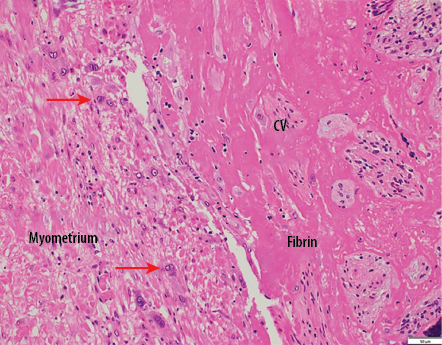 Figure 5: Chorionic villi (CV) implants directly onto myometrial fibers with no intervening decidua. Only extravillous trophoblastic cells (red arrows) and fibrin separate villi from myometrial fibers with no intervening decidua. Hematoxylin and eosin staining, magnification = 200 ×.
Figure 5: Chorionic villi (CV) implants directly onto myometrial fibers with no intervening decidua. Only extravillous trophoblastic cells (red arrows) and fibrin separate villi from myometrial fibers with no intervening decidua. Hematoxylin and eosin staining, magnification = 200 ×.
Discussion
The incidence of PAS has been rising globally for the past few decades, which is thought to be due to the increased rate of cesarean section. The odds ratio for PAS in the presence of one, two, or three prior cesarean section deliveries were 2.6%, 4.9%, and 7.7%, respectively.3
Other independent risk factors for PAS include placenta previa diagnosed before birth. Other predisposing factors include prior myomectomy, curettage, and multiparity.4 According to a large prospective cohort study by the Utah Health Sciences Center on the risk factors for PA, the number of PAS reported was likely greater in higher parity women. These women were older than those without PAS. There were 83 out of 196 cases of PAS reported in a parity greater than three, which accounts for almost half of the cases.4 There was also an increased risk of PAS associated with older maternal age in women without a previous cesarean delivery. In other studies, comparison of primigravida women with advanced maternal age of > 40 years carried 19-fold higher odds of PAS compared with mothers aged < 30.3,5 This is correlated to our case report as there is a high chance of acquiring PAS in multiparity and advanced maternal age.
It is unclear how normal placenta location as in this case, is predisposed to PAS. It could be related to multiparity, as with repeated deliveries, predispose to scarring or poor vasculature of the uterus, which is associated especially with increasing age.6 Later, may predispose to invasion, even in women without a prior cesarean section as in this case.
There are three variants of PAS identified, which are accreta, increta, and percreta. Accreta is when the placental tissue adheres to the decidual surface of the myometrium. In increta, the placenta villi invade more deeply within the myometrium, and percreta is when CV penetrate through the uterine serosa and may invade surrounding organs such as the bladder.7 The presence of PAS is associated with major complications and maternal morbidity including massive blood transfusion, urinary tract injury, hysterectomy, admission to intensive care unit, sepsis, deep vein thrombosis, pulmonary embolism, and even prolonged hospital stay.7,8 In terms of neonatal outcome, studies have shown that PAS may not have a direct effect, therefore antenatal fetal surveillance is not necessary unless indicated.9
In normal pregnancy, the decidualized endometrial stroma is the site of placental separation from the uterine wall by shearing action between the contracting myometrium and the non-contracting placenta. In PAS, the absence of decidua prevents separation, thus leading to a clinically adherent placenta and subsequently causing massive hemorrhage. The exact reason for this is still unknown, however, in previous cesarean section, the possible pathophysiology is thought to be due to an absence or deficiency of Nitabuch’s fibrinoid layer or the spongiosum layer of the decidua which results in affixing of the CV to the myometrium.8
Several studies have suggested that inordinate trophoblast invasion may contribute to PAS. Normal implantation of the placenta involves invasion of the uterine wall by two special subdivisions of extravillous trophoblast to establish critical tissue connection in the development of placenta-uterine intersection; the interstitial trophoblast (invading through endometrium and superficial one-third of the myometrium) and the endovascular trophoblast (invading and remodeling the maternal spiral arterioles). This later causes the defeat of the smooth muscles in the arterial wall, resulting in vessels with dilated lumina and a hyalinized wall containing endovascular trophoblast.9 Benirschke and Kaufmann suggest that this is the consequence of a failure of refashioning of the endometrium or/and decidual basalis after repair during cesarean section.10 Histology usually shows that the trophoblast has invaded the myometrium without intervening decidua.
It is believed that in PAS there is defective interaction between maternal tissues and migratory trophoblast in the early stages of placentation, together with the development of an abnormal uteroplacental circulation resulting in profound trophoblast invasion into the uterus and resulting in PAS.11
In response to the high maternal morbidity, there should be a prompt diagnostic approach to PAS including transabdominal ultrasound (US), which is a non-invasive procedure. There are sonographic features suggestive of PAS including the irregular shape of placental lacunae within the placenta often containing turbulent flow, thinning of the myometrium overlying the placenta, and loss of ‘clear space/zone’ between the uterus and placenta. The placenta also can bulge into the bladder with intact serosa causing bladder wall interruption and increased vascularity of the uterine serosa-bladder interface, and color Doppler US showed uterovesical hypervascularity.12
Magnetic resonance imaging may be used as a complement to the US in assessing the depth of invasion and lateral extension of myometrial invasion, especially with posterior placentation and/or suggesting parametrial invasion. The features include abnormal uterine bulging, dark intraplacental bands on T2-weighed imaging, heterogenous signal intensity within the placenta, disorganized vasculature of the placenta, and disruption of the uteroplacental zone.12,13
Management of PAS involves a planned cesarean hysterectomy between 35 to 36 weeks of gestation to provide a balance between fetal maturity and risk for an emergency delivery. Cesarean hysterectomy with the placenta left in situ is preferable compared to attempting separation of the placenta from the uterine wall. This significantly reduced the short-term morbidity such as massive postpartum hemorrhage that later predisposes to disseminated intravascular coagulopathy.13
On the other hand, in selected cases, conservative management by leaving the placenta in situ versus the extirpative method is essential to attempt to decrease the risks of severe maternal morbidity during cesarean delivery.14,15 Forcibly removing an invasive placenta with placental villi that have invaded the deep uterine vasculature increases the risks of massive obstetric hemorrhage and the need for salvation hysterectomy. Uncontrolled bleeding will lead to coagulopathy and will also complicate the surgical procedure, increasing the risk of injuries mainly to the bladder and ureters, and their possible long-term complications such as vesicouterine fistula.
Conclusion
Advanced age and multiparity represent unique risk factors for PAS. Furthermore, undiagnosed PAS in a normally-situated placenta without previous uterine surgery may lead to postpartum hemorrhage, which subsequently increases the risk of morbidity and mortality. A high index of suspicion with early initiation of cesarean hysterectomy in this case reduces the risk of massive postpartum hemorrhage and coagulopathy.
Disclosure
The authors declared no conflicts of interest. Written consent was obtained from the patient.
references
- 1. Fitzpatrick KE, Sellers S, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. Incidence and risk factors for placenta accreta/increta/percreta in the UK: a national case-control study. PloS One 2012 Dec 27;7(12):e52893.
- 2. Bowman ZS, Eller AG, Bardsley TR, Greene T, Varner MW, Silver RM. Risk factors for placenta accreta: a large prospective cohort. Am J Perinatol 2014 Oct;31(09):799-804.
- 3. Majeed T, Waheed F, Mahmood Z, Saba K, Mahmood H, Bukhari MH. Frequency of placenta previa in previously scarred and non scarred uterus. Pak J Med Sci 2015;31(2):360-363.
- 4. Jafari RM, Najafian M, Barati M, Saadati N, Jalili Z, Poolad A. Comparison of uterine preservation versus hysterectomy in women with placenta accreta: a cross-sectional study. Int J Reprod Biomed 2022 Oct 10;20(9):739-744.
- 5. Kayabasoglu F, Guzin K, Aydogdu S, Sezginsoy S, Turkgeldi L, Gunduz G. Emergency peripartum hysterectomy in a tertiary Istanbul hospital. Archives of Gynecology and Obstetrics 2008 Sep;278:251-256.
- 6. Kiondo P, Wandabwa J, Doyle P. Risk factors for placenta praevia presenting with severe vaginal bleeding in Mulago hospital, Kampala, Uganda. Afr Health Sci 2008 Mar;8(1):44-49.
- 7. Farquhar CM, Li Z, Lensen S, McLintock C, Pollock W, Peek MJ, et al. Incidence, risk factors and perinatal outcomes for placenta accreta in Australia and New Zealand: a case–control study. BMJ Open 2017 Oct 1;7(10):e017713.
- 8. Balayla J, Bondarenko HD. Placenta accreta and the risk of adverse maternal and neonatal outcomes. J Perinat Med 2013 Mar;41(2):141-149.
- 9. Tantbirojn P, Crum CP, Parast MM. Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta 2008 Jul;29(7):639-645.
- 10. Benirschke K, Kaufmann P. Pathology of the human placenta. 4th ed. New York: Springer; 2000.
- 11. Goh WA, Zalud I. Placenta accreta: diagnosis, management and the molecular biology of the morbidly adherent placenta. J Matern Fetal Neonatal Med 2016;29(11):1795-1800.
- 12. Comstock CH, Love JJ Jr, Bronsteen RA, Lee W, Vettraino IM, Huang RR, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol 2004 Apr;190(4):1135-1140.
- 13. Jauniaux ER, Alfirevic Z, Bhide AG, Belfort MA, Burton GJ, Collins SL, et al. Placenta praevia and placenta accreta: diagnosis and management: green-top guideline no. 27a. BJOG 2018;126(1):e1-e48.
- 14. Sentilhes L, Merlot B, Madar H, Sztark F, Brun S, Deneux-Tharaux C. Postpartum haemorrhage: prevention and treatment. Expert Rev Hematol 2016 Nov;9(11):1043-1061.
- 15. Sentilhes L, Vayssière C, Deneux-Tharaux C, Aya AG, Bayoumeu F, Bonnet MP, et al. Postpartum hemorrhage: guidelines for clinical practice from the French college of gynaecologists and obstetricians (CNGOF): in collaboration with the French society of anesthesiology and intensive care (SFAR). Eur J Obstet Gynecol Reprod Biol 2016 Mar;198:12-21.