Latent autoimmune diabetes in adults (LADA) is estimated to account for 2–14% of all cases of diabetes in adults.1,2 Often misdiagnosed as type 2 diabetes mellitus (T2DM), it causes inefficient and delayed glycemic control leading to enhanced diabetes-related mortality and morbidity.3
The fourth edition of Diabetes Mellitus Management Guidelines of Oman (2021)4 mentions one form of diabetes as ‘slowly evolving immune-mediated diabetes of adults’, though the term LADA is not referred for this category, nor screening recommendation is mentioned. A note suggests that antibody testing should be done when there is uncertainty in the diagnosis of type 1 diabetes mellitus (T1DM) by clinical presentation.4
According to the Immunology of Diabetes Society’s criteria to diagnose LADA,5 the patient should be: (a) aged ≥ 30 years; (b) positive for at least one of the four antibodies commonly seen in T1DM, namely islet cell antibodies, anti-glutamic acid decarboxylase (GAD) autoantibodies, insulinoma associated-2 autoantibodies, and insulin autoantibodies; and (c) free of insulin treatment for the first six months after the initial diagnosis.
On initial presentation, especially in young adults, it is important to consider the possibility of LADA and know how to distinguish its symptoms and history from those of T1DM and T2DM [Table 1].
Table 1: Comparative characteristics of type 1 diabetes mellitus (T1DM), latent autoimmune diabetes in adults (LADA), and type 2 diabetes mellitus (T2DM).6
|
Age of onset
|
Childhood or adulthood
|
Adulthood
|
Adulthood
|
|
Metabolic syndrome
|
Similar to the general population
|
Similar to the general population
|
Present in 80–90% of cases
|
|
Ketoacidosis
|
Frequent
|
Infrequent
|
Absent
|
|
Autoimmunity
|
Present (ICA predominates)
|
Present (anti-GAD predominates)
|
Absent
|
ICA: islet cell autoantibodies; GAD: glutamic acid decarboxylase.
However, the above criteria turned out to be insufficient to diagnose LADA. This is because LADA is a multiplex disorder with a few patients manifesting high antibody titers, lower body mass index (BMI), and progressing rapidly to insulin requirement, while others have low antibody titers with features of insulin resistance such as higher BMI and progress more slowly to insulin requirement.3 LADA with high BMI is also referred to as ‘double diabetes’, and these patients are also likely to have a family history of T2DM with or without the clinical features of insulin resistance.7
In a retrospective study, Fourlanos et al,8 proposed a five-point LADA clinical screening tool consisting of (a) age at onset < 50 years; (b) acute symptoms; (c) BMI < 25 kg/m2; (d) a history of autoimmune disease; and (e) a family history of diabetes. This tool had a sensitivity of 90% and specificity of 71% in identifying LADA patients if they had at least two of the five clinical features. In cases where only one or none of these features were present, a negative predictive value of 99% was given.8
Case Report
A 34-year-old Omani woman with a strong family history of diabetes was detected to have gestational diabetes in the first pregnancy, which reverted to normoglycemia after the delivery of a healthy baby. No pharmacological intervention was required post-partum. During her second pregnancy, she was normoglycemic but had an episode of suspected hyperemesis gravidarum with mild hyperglycemia and ketosis, which was managed conservatively. During discharge, she was advised to monitor her blood glucose. The patient went on to have a spontaneous vaginal delivery of a healthy second child.
Six weeks postnatal, she was confirmed to have diabetes, due to glycated hemoglobin (HbA1c) at 9.1% and two-hour oral glucose tolerance at 12.1 mmol/L. The diabetes was initially managed with diet control and lifestyle changes.
In later follow-ups, her HbA1c remained uncontrolled; hence metformin 1000 mg twice a day was started which was intensified with gliclazide 60 mg once before breakfast. Despite the two oral therapies, hyperglycemia persisted; hence she was referred to a specialized center where sitagliptin 100 mg once a day was added.
The patient was seen in the diabetes clinic of the Royal Oman Police Hospital with persistent hyperglycemia and a weight loss of 5 kg in three months. At presentation, she was taking the above three medications except for sitagliptin, which had been reduced to 50 mg daily.
Lab tests revealed fasting blood glucose level at 18.1 mmol/L, HbA1c at 15%, and urine ketones ++. There was no microalbuminuria; kidney functions and other laboratory findings were within the normal limits.
On clinical examination, she had a BMI of 24, her vitals were normal, and she was hemodynamically stable except for mild dehydration. An initial impression of T1DM was made. She was treated for hyperglycemia with insulin protocol, and the dehydration was corrected with intravenous normal saline.
After stopping metformin, gliclazide, and sitagliptin, blood samples were sent for testing c-peptide and anti-GAD. A basal insulin in the form of insulin detemir was initiated. Following the patient’s dietary regimen, her main meal being lunch, with no breakfast and no (or light) dinner, a pre-lunch rapid-acting insulin was included in the regime. She was counseled about the need to add in a pre-dinner rapid acting insulin or switching to premix insulin twice a day.
Two weeks later, she was reviewed in the clinic with the following results: fasting blood glucose of 9.5 mmol/L; fasting c-peptide of 1.68 pmol/L (260–1270 pmol/L); and a positive anti-GAD. A diagnosis of LADA was made in concordance with clinical examination and biochemical parameters along with the available guidelines for LADA. She was started on a twice-daily premix regime of insulin aspart and protamine (30:70).
On later follow-ups, the patient’s self-monitored blood glucose level showed remarkable improvement. With gradual up-titration, her condition improved symptomatically and biochemically, including a marginal weight gain of 1.4 kg.
Discussion
The prevalence of T2DM is almost 90–95% in patients with diabetes, while the prevalence of LADA can be 10% in patients with a phenotypic of T2DM, thus indicating a much larger representation than in patients with T1DM.9 Diagnosis of LADA consists of testing for autoantibodies, usually positive for at least one of the four antibodies commonly seen in T1DM. Anti-GAD might not be detectable in early stages but can present over a period of time; hence undetectable anti-GAD level alone cannot rule out LADA. A family history of diabetes is usually present. A consensus statement from an international expert panel is visually presented in Figure 1.
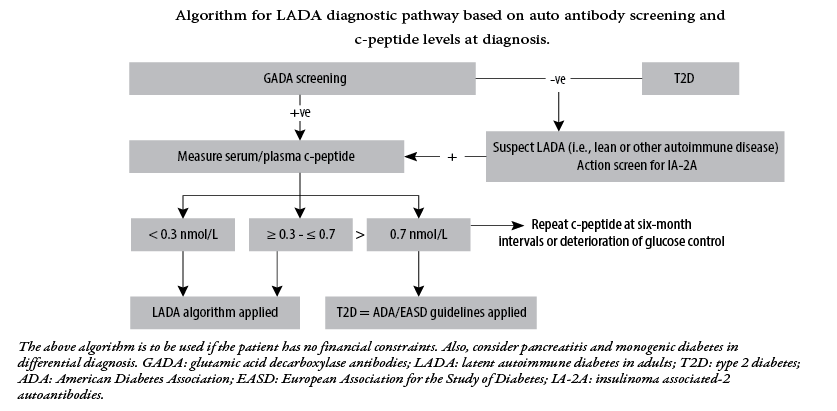 Figure 1: Management of latent autoimmune diabetes in adults: a consensus statement from an international expert panel.10
Figure 1: Management of latent autoimmune diabetes in adults: a consensus statement from an international expert panel.10
Management of LADA includes diet, lifestyle, and insulin. Obese LADA (double diabetes) patients may require an individualized regimen of calorie-restricted diet and physical activity. It is also recommended to avoid insulin secretagogues such as glipizide. This is because the stimulation of insulin by these drugs promotes increased autoantigen expression, which accentuates the ongoing autoimmune process.11 Metformin can be used in obese LADA patients, but insulin remains the treatment of choice.
Clinicians should also be aware that all diabetic patients including those with LADA have a similar risk of developing cardiovascular and cerebrovascular diseases.12 In addition, LADA patients have a higher risk than T2DM patients for certain autoimmune conditions such as thyroid disease.13
Conclusion
Definitive guidelines in the management of LADA are not yet available, but early diagnosis of LADA helps patients achieve better glycemic control and avoid the long-term complications of diabetes. Timely adoption of insulin therapy also appears to benefit these patients.
Disclosure
The authors declared no conflicts of interest. Informed written consent was taken from the patient.
references
- 1. Buzzetti R, Tuomi T, Mauricio D, Pietropaolo M, Zhou Z, Pozzilli P, et al. Management of latent autoimmune diabetes in adults: a consensus statement from an International Expert Panel. Diabetes October 2020; 69(10):2037-2047.
- 2. Pozzilli P, Pieralice S. Latent autoimmune diabetes in adults: current status and new horizons. Endocrinol Metab (Seoul) 2018 Jun;33(2):147-159.
- 3. Rajkumar V, Levine SN. Latent autoimmune diabetes. StatPearls Publishing. 2023 [cited 2023 April 17]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557897/.
- 4. Lutgens MW, Meijer M, Peeters B, Poulsen ML, Rutten MJ, Bots ML, et al. Easily obtainable clinical features increase the diagnostic accuracy for latent autoimmune diabetes in adults: an evidence-based report. Prim Care Diabetes 2008 Dec;2(4):207-211.
- 5. Naik RG, Brooks-Worrell BM, Palmer JP. Latent autoimmune diabetes in adults. J Clin Endocrinol Metab 2009 Dec;94(12):4635-4644.
- 6. Pollak F, Vásquez T. Diabetes autoinmune (latente) del adulto. Rev Med Chil 2012 Nov;140(11):1476-1481.
- 7. Kietsiriroje N, Pearson S, Campbell M, Ariëns RA, Ajjan RA. Double diabetes: a distinct high-risk group? Diabetes Obes Metab 2019 Dec;21(12):2609-2618.
- 8. Fourlanos S, Perry C, Stein MS, Stankovich J, Harrison LC, Colman PG. A clinical screening tool identifies autoimmune diabetes in adults. Diabetes Care 2006 May;29(5):970-975.
- 9. Qi X, Sun J, Wang J, Wang PP, Xu Z, Murphy M, et al. Prevalence and correlates of latent autoimmune diabetes in adults in Tianjin, China: a population-based cross-sectional study. Diabetes Care 2011 Jan;34(1):66-70.
- 10. Buzzetti R, Tuomi T, Mauricio D, Pietropaolo M, Zhou Z, Pozzilli P, et al. Management of latent autoimmune diabetes in adults: a consensus statement from an international expert panel. Diabetes 2020 Oct;69(10):2037-2047.
- 11. Poudel RR. Latent autoimmune diabetes of adults: from oral hypoglycemic agents to early insulin. Indian J Endocrinol Metab 2012 Mar;16 (Suppl1):S41-S46.
- 12. Myhill P, Davis WA, Bruce DG, Mackay IR, Zimmet P, Davis TM. Chronic complications and mortality in community-based patients with latent autoimmune diabetes in adults: the Fremantle diabetes study. Diabet Med 2008 Oct;25(10):1245-1250.
- 13. Murao S, Kondo S, Ohashi J, Fujii Y, Shimizu I, Fujiyama M, et al. Anti-thyroid peroxidase antibody, IA-2 antibody, and fasting c-peptide levels predict beta cell failure in patients with latent autoimmune diabetes in adults (LADA)–a 5-year follow-up of the Ehime study. Diabetes Res Clin Pract 2008 Apr;80(1):114-121.