Breast carcinoma is the most prevalent cancer in women worldwide.1 The global burden of breast cancer, which saw 19.3 million new cases in 2020, is expected to rise to 28.4 million cases by 2040 with a 47% rise from 2020.2,3 Breast carcinoma in African and African-American women is characterized by hormone receptor triple-negative tumors, late presentation, younger age, advanced stage, and higher grade,4 leading to worse prognoses compared to Caucasian western women.5 For optimal management, it is essential to have an accurate diagnosis of the state of the hormone receptors including the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2).6 Breast carcinomas are classified into four major distinct immunohistochemical (IHC)/molecular subtypes based on these intrinsic biologic factors using the following IHC markers: luminal A (ER-positive, PR-positive, and HER-2 negative); luminal B (ER-positive, PR-positive, and HER-2 positive); HER-2 over-expression (ER-negative, PR-negative, and HER-2 positive); and triple-negative/basal-like (ER-negative, PR negative, and HER-2 negative).6
Oncologists have also classified breast cancers based on therapy choices using immunohistochemistry as a stand-in: (a) cancers with ER and treated with chemotherapy; (b) cancers having HER-2/neu and treated with trastuzumab or lapatinib; and (c) cancers having negative hormone receptors for ER, PR, and HER-2 (i.e., the ‘triple-negative subgroup’) where chemotherapy is the only treatment option.7 The triple-negative subtype is reported to disproportionately impact young African-American women, and exhibit aggressive clinical behavior and high histopathological grades.7
We aimed to study the immunohistochemical and clinicopathological characteristics of breast carcinoma in the Nigerian city of Ilorin, including their histopathological types and grades, staging, and immunohistochemical subtypes.
Methods
We conducted a retrospective assessment of patients with breast carcinoma who received a diagnosis at the Department of Pathology, University of Ilorin Teaching Hospital, , Kwara State, Nigeria between 2012 and 2019.
The patients’ request cards were used to retrieve personal information, tumor size, histopathological diagnosis, Nottingham grading system, lymph node status, and immunohistochemical diagnosis (hormone receptor status and human epidermal growth factor findings). Medical records were used to extract the history of co-morbidities, history of neoadjuvant chemotherapy, local symptoms like breast lumps, deformed breast shapes, tumor sizes, puckering skin changes, peau d'orange, ulcers, fixation to chest wall structures, andtreatment received.
Our inclusion criteria restricted the participants to women with invasive breast carcinoma diagnosis whose full pathological data was available (including tumor grade, tumor size, number of harvested positive lymph nodes, clinical staging, and type of surgery performed). Patients with incomplete data were excluded from the study.
Our hospital procedure required all harvested surgical biopsies and mastectomies to be immediately subjected to fixation in 10% neutral buffered formalin in a labeled container and sent to the pathology department for study. There, the specimens were grossed by determining the size, color, consistency, weight, and dimension of the tumor. Following tissue processing, embedding, and routine hematoxylin and eosin staining, representative tumor tissue section slides were prepared and used to make histopathological diagnoses. The Nottingham composite histopathologic grading scheme was used to access the histopathological grades (Elston-Ellis modification of Scarf-Bloom-Richardson-Elson grading system).8 The WHO classification system from 2012 was used to assess the various histopathological types.9 The TNM system, endorsed by Union for International Cancer Contro and the American Joint Committee on Cancer and End Results Reporting, was used to assess tumor staging.10
The immunohistochemistry procedure with avidin-biotin complex, also known as the Avidin biotin Immunoperoxidase technique, was employed on the formalin-fixed paraffin-embedded tissue blocks used for this study. Therefore, the slides on ER and PR were assessed using nuclear reactivity and the slides on HER-2 using membrane reactivity. Cells with complete membrane staining that appear brownish for HER-2 were regarded as positive. Cells with distinct brown colors in the nuclei for ER and PR were also considered positive. However, immunostained negative tumor cells were shown as bluish. Non-specific binding/brown artifacts on cells and connective tissue were ignored.
The staining intensity of immunohistochemical reactions was graded and scored as per the Allred scoring guidelines. The percentage of tumor nuclei stained and the intensity of staining were the two components that make up the Allred grading system (for ER and PR), where a score ≥ 1 indicates that both proportion and intensity are positive. HER-2/neu was assessed using the Dako Herceptest scoring guideline, yielding results of 0, +1, +2, and +3. Sections with scores of +3 were viewed as positive, whereas those with scores of 0 and +1 were viewed as negative, and scores of +2 as equivocal (requiring in-situ hybridization studies for confirmation).
The SPSS Statistics (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) was used to analyze the data. The data was displayed in tables, charts, and photomicrographs. For quantitative variables, data was described with the mean SD, and for qualitative variables, statistical significance was defined as p < 0.05.
This study followed the ethical principles of the Helsinki Declaration on human subjects in biomedical research. Information about the patients’ identities and personal health was kept private. The ethical approval for the study was obtained from the University of Ilorin Teaching Hospital Ethical Committee with registration number NHREC/02/05/2010 dated 28 December 2016.
Results
This study examined specimens from N = 113 breast carcinoma patients, which included 36 surgery biopsies (31.9%) and 77 mastectomies (68.9%).
The average patient age was 52.1±12.1 (range = 30–80) years. There were more patients in their forties and fifties than in the other age groups [Table 1]. Invasive ductal carcinoma not otherwise specified (NOS) accounted for 107 of 113 (94.7%) patients. Grade I breast cancers were detected in 20 (17.7%) cases in the Nottingham grading scheme, while grade III and grade II cancers numbered 47 (41.6%) and 46 (40.7%), respectively [Table 2].
Table 1: Age at presentation (in years) of patients with breast cancer (N = 113).
|
< 40
|
20
|
17.7
|
|
40 – 49
|
37
|
32.7
|
|
50 – 59
|
34
|
30.1
|
|
60 – 69
|
10
|
8.8
|
|
≥ 70
|
12
|
10.6
|
|
Mean ± SD
|
52.1 ± 12.1
|
|
Table 2: Histopathological type and grade of breast carcinoma (N = 113).
|
Invasive ductal carcinoma
|
107
|
94.7
|
|
Metaplastic carcinoma
|
1
|
0.9
|
|
Mucinous carcinoma
|
3
|
2.7
|
|
Papillary carcinoma
|
1
|
0.9
|
|
Plasmacytoma
|
1
|
0.9
|
|
Histopathological grade
|
|
1
|
20
|
17.7
|
|
2
|
46
|
40.7
|
Lymph nodes in the 83 mastectomy specimens were investigated and divided into three groups, (a) N0 (where the cancer has not spread to the lymph nodes), (b) N1 (metastatic carcinoma has affected 1–3 lymph nodes), and (c) N3 (significant metastatic carcinoma in lymph nodes). N0 was found in 35 of 83 (42.2%) lymph node samples, N1 in 47 (56.6%), and N3 in one (1.2%) [Table 3]. The tumor diameters ranged from 3.2–16.0 (6.8±3.2 cm). Most patients (75.9%) had tumors > 5.0 cm [Table 3].
Table 3: Specimen type, tumor size, and lymph node of women with breast cancer.
|
Type of specimen
|
|
Biopsy
|
36
|
31.9
|
|
Mastectomy
|
77
|
68.1
|
|
Tumor size (cm)
|
83
|
|
|
< 2
|
0
|
0.0
|
|
2–5
|
20
|
24.1
|
|
> 5
|
63
|
75.9
|
|
Mean ± SD
|
6.8 ± 3.2
|
|
|
Range
|
0 – 16
|
|
|
Lymph node
|
|
N0
|
35
|
42.2
|
|
N1
|
47
|
56.6
|
All cases were subjected to immunohistochemical evaluation using monoclonal ER, PR, and HER-2 antibodies. Most tumor cells (72 of 113; 63.7%) were ER-negative, and the remaining 41 (36.3%) were ER-positive. In contrast, 81 (71.7 %) were PR-negative and the remaining 32 (28.3%) were PR-positive. HER-2 positivity was found in (47 of 113 (41.6 %) tumor cells [Table 4 and Figure 1]. Additionally, triple-negative (ER-/PR-/HER-2-) accounted for 48 (42.5%), luminal A (ER+/PR+/HER-2-) for 19 (16.8%), luminal B (ER+/PR+/HER-2+) for 23 (20.4%), and HER-2 enriched (ER-/PR-/HER-2+) for 23 (20.4%) cases [Figures 1 and 2]. Notably, 52 (46.0%) patients were in stage T3, 29 (25.7%) were in stage T4, and one (0.9%) patient was in stage T1 [Table 5]. We found that the predominant locoregional symptom of breast carcinoma was lumps as found in 105 (92.9%) patients, followed by deformity of the breast (78; 69.0%) and ulceration (21; 18.6%) [Table 6].
Table 4: Immunopositivity of estrogen receptor, progesterone receptor, and HER-2 biomarkers.
|
ER
|
41 (36.3)
|
72 (63.7)
|
113 (100)
|
|
PR
|
32 (28.3)
|
81 (71.7)
|
113 (100)
|
HER-2: epidermal growth factor receptor-2; ER: estrogen receptor;
PR: progesterone receptor.
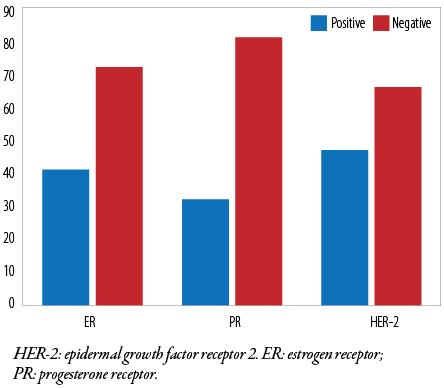 Figure 1: Immunohistochemical expression of ER, PR, and HER-2 biomarkers.
Figure 1: Immunohistochemical expression of ER, PR, and HER-2 biomarkers.
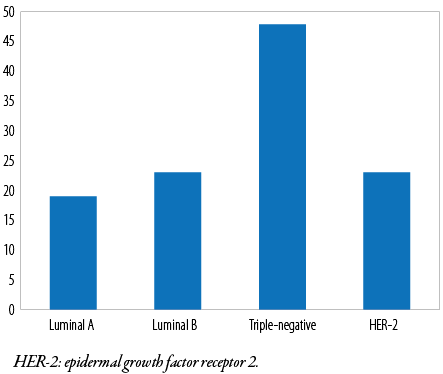 Figure 2: Immunohistochemical subtypes of breast carcinoma.
Figure 2: Immunohistochemical subtypes of breast carcinoma.
Table 5: Staging of breast carcinoma (N = 103).
|
T1
|
1
|
0.9
|
|
T2
|
21
|
18.6
|
|
T3
|
52
|
46.0
|
T: tumer size.
Table 6: Presenting symptoms of breast cancer patients* (N= 113).
|
Lump
|
105 (92.9)
|
8 (7.1)
|
|
Deformity of the breast
|
78 (69.0)
|
35 (31.0)
|
|
Ulceration
|
21 (18.6)
|
92 (81.4)
|
|
Lump and deformity
|
57 (50.4)
|
56 (49.6)
|
*Multiple symptoms may have presented in the same patient.
Discussion
This study sought to determine the age of breast carcinoma patients, their histopathological types and grades, staging, and immunohistochemistry subtypes. It also aimed to analyze the clinicopathological and immunohistochemical characteristics of breast carcinoma in Ilorin, Nigeria.
The age range of our patients was 30–80 years and the most represented age group (32.7%) was 40–49 years. The mean age at presentation was 52.1±12.1 years. These are comparable to the previous findings at the same center.11,12 Our results are also consistent with those from other parts of Nigeria.13,14,15,16 The peak age at the fifth decade has also been endorsed by research in Nigerian cancer registries at Ibadan, Lagos, and Calabar.12,13 Research from Ghana, Egypt, Sudan, Saudi Arabia, Malaysia, and Eastern Europe also endorse a similar the fifth-decade peak.17–22
At 94.7%, invasive ductal carcinoma was the most prevalent histological type of breast cancer NOS among our patients, similar to the 82.6% prevalence reported by an earlier study at the same center.11 Comparable findings have also emerged from Ibadan, Calabar, Lagos, and Sokoto.16,23,24 The notable exception was a study from Uyo (Nigeria) which reported much lower prevalence (53.0%) of invasive ductal carcinoma NOS than most other studies including ours.25 Studies in other African countries have also yielded similar prevalence as ours: Sudan (90.0%), Cameroon (90.0%), Ghana (75.8%), Tanzania (78.0%), and Tunisia (87.0%).26–30 Comparable prevalence of invasive ductal carcinoma have been reported from Iraq (93.5%) and India (88.0%) as well.31,32
Mucinous carcinoma represented 2.7% of the cases in this study, compared to the lower prevalence reported from Oshogbo and Lagos (1.2–2.5%).25,33 Results from other parts of Nigeria have been diverse.9,20,30 Kano in northwestern Nigeria reported a significantly higher (6.9%) prevalence of mucinous carcinoma than most other Nigerian studies including ours.34 Data from other African nations revealed varied prevalence of mucinous carcinoma ranging from 2% to 9.8%.17,31,35
The prevalence of metaplastic carcinoma in our study was 0.9%, and similar findings elsewhere ranged from 0.19% to 2.4%.12,33,36
In our study, Nottingham grade I breast carcinoma accounted for 17.7% of cases compared to 12.7% in Lagos, 9.4% in Ibadan, and 5.9% in Jos.23,37,38 Dramatically, high prevalence of 27.3% of grade I breast carcinoma was reported from Kano in northwest Nigeria.39 The rates of grade I breast carcinoma in other African nations have been reported in Sudan, Tunisia, Ghana, and Tanzania at 1.0%, 9.0%, 14.8%, and 25.0%, respectively.20,26,29,30 Outside Africa, a prevalence of 4.2% has been reported in Pakistan, 17.4% in Iraq, and 29.0% in the USA.31,40,41 Geoclimatic, ethnic, and lifestyle differences might be hypothesized to be responsible for much of the observed variations in the prevalence of different types of breast cancer.
In this study, Nottingham grade II breast cancer represented 40.7% of all cases, vis-à-vis Ibadan (44.3%), Lagos (48.0%), Kano (33.4%), Jos (23.5%), and Calabar (23.5%).17,27,34,37,38 The prevalence of grade II breast carcinoma in other countries was widely variable: Ghana (31.5%–39.7%),17,28 Iran (36.2%), the USA (41.4%), and Pakistan (75.0%).28,37,38 The discrepancy in histopathologic grades noted in the various study populations may be due to the Nottingham histopathologic grade system’s interobserver variability, and variations in the disease’s demographic distribution.
Nottingham grade III breast carcinoma had a prevalence of 41.6% among the participants in this study. Prevalence rates reported from different parts of Nigeria include 29.7% of cases in Ilorin12 and 15.6% in Ibadan,37 7.1% in Kano, 66.7% in Calabar, and 70.6 % in Jos.16,34,38 Nottingham grade III prevalence in other African nations were 30.3% in Cameroon, 35.1% in Tunisia, 53.7% in Ghana, and 68.0% in Sudan.17,26,27,30 Across the world, the prevalence was 20.3% in Pakistan, 30.3% in the USA, and 46.4% in Iran.31,40,41 Different types of biopsies (tru-cut, incisional, and excisional) used for assessment and interobserver variability in the assessment of breast carcinoma using the Nottingham histopathologic grade system may partly have led to the discrepancies of findings in the surveys in Nigeria, other African nations, and developed countries. Due to the minimal amount of breast tissue compared to incisional or excisional breast biopsies, tru-cut biopsies tend to downgrade breast carcinoma. The accuracy of diagnoses might also be affected by geopolitical and socioeconomic factors.
The size of the tumor, clinical stage, and lymph node metastases are significant independent prognostic variables for invasive breast carcinoma. A sizable proportion of women with breast carcinoma in this study presented with lymph node involvement, matching stages 3 and 4, and had tumor sizes > 5 cm. Our results are comparable to those from regional and international studies.34,38,42–44
When filling out the pathology request form, many patients did not have the proper documentation of their clinical staging, which could affect the accuracy of the database and subsequent follow-up and management. To properly prognosticate patients, the pathologist should take careful note of the tumor’s size and thoroughly dissect the axillary tail fat. Due to false positives and false negatives—such as reactive follicular hyperplasia and sinus histiocytosis of the nodes—the clinical assessment of lymph node status is inaccurate (e.g., lymph nodes with small metastatic deposits). Consequently, a biopsy is required for a precise evaluation and diagnosis. The 10-year disease-free survival rate is close to 70% to 80% with no nodal involvement; the rate drops to 35% to 40% with one to three positive nodes, and 10% to 15% with more than ten nodes positive.45 In addition, prior studies only examined the lymph nodes for the presence of malignancy without stratifying and specifying the number of positive lymph nodes for staging.45 The majority of patients in this study had advanced tumors that were > 5 cm, consistent with past studies in Nigeria and other Sub-Saharan African nations.33,36,40,43 Lack of coordinated breast cancer screening, political apathy, low awareness, absence of established screening programs using ultrasound and mammography, poverty, sociocultural practices, and religion may all contribute to this. Indeed, in low-resource African countries such as Nigeria, the immunohistochemistry investigation of breast carcinomas faces severe barriers. These include patients’ financial constraints to afford the cost of investigations, shortage of qualified technical staff, disruptions in the supply of essentials such as monoclonal antibodies, detection kits, and charged slides, as well as infrastructure limitations including subpar tissue storage facilities. It is essential that these barriers are overcome so that the major problem of breast cancer in African women is studied and mitigated.
Breast carcinoma tumor cells in this research had immunohistochemistry reactivity for ER, PR, and HER-2 at rates of 36.3%, 28.3%, and 41.6%, respectively. A retrospective study conducted in Nigeria and Senegal that included 507 patients diagnosed with breast carcinoma among African Americans of Nigerian and Senegalese origin between 1996 and 2007 showed ER, PR, and HER-2 at rates of 24.0%, 20.0%, and 17.0% respectively; however, this contrasts with a study conducted in the University of Ibadan and University of Calabar, a few years ago, that showed 27.0%, 16.0%, and 30.0% for ER, PR, and HER-2, respectively.4,44 While Agbo et al,46 in Sokoto discovered that ER, PR, and HER-2 were 47.8%, 41.3%, and 43.5%, respectively for immunostained breast carcinoma tumor cells, the corresponding values from Uyo were 18.0%, 14.8%, and 32.8%, respectively.45 In Ibadan, the ER, PR, and HER-2 expression was 65.1%, 54.7%, and 79.7%, respectively. A USA study among Caucasians found that ER, PR, and HER-2 expression was 45.0%, 37.0%, and 32.0%, respectively.37,40,47 Such wide variations in the immunoreactivity values in studies above might be due to limitations in some studies. These may include the use of archival paraffin-fixed embedded tissue blocks with antigen degradation due to poor storage or suboptimal adherence to prescribed guidelines for handling and storing breast specimens in some studies.48
The immunohistochemical subtypes of breast carcinoma recorded in this study were 16.8%, 20.4%, 20.4%, and 42.5% for luminal A, luminal B, HER-2 enriched, and triple-negative, respectively. These results differed from a previous study in our center which were 20.0%, 11.0%, 30.0%, and 25.0%, respectively.44 The results were equivalent to those of a study carried out in Sokoto, Senegal, and Nigeria’s six geopolitical zones.4,46 However a study among Nigerians who were African Americans and Senegalese at six geopolitical zones where University College Hospital in Ibadan was used as coordinating center revealed that the hormonal expression in patients from African-American patients was equivalent to that of Caucasians and people from other parts of the world.37,40,49–54 The differences observed among racial groups and geographical areas could be explained by tumor biology, various antibody clones, antigen retrieval standard operating procedure, and bias in the use of the Allred and Herceptest scoring system.
The chief limitation of this study is its retrospective nature; the principal investigator lacked control over the historical patient data, and preanalytical/analytical tissue handling variables including those related to standardized cold ischaemic time in the operating room, fixative composition, and fixation time. Additionally, due to a lack of resources, the breast carcinoma cases with HER-2 membrane staining scores of 2+ could not be subjected to in-situ hybridization tests such as fluorescent in-situ hybridization for further characterization.
The next step will be to carry out a prospective study where the lead researcher will oversee these variables. Along with performing molecular biological studies like fluorescent in-situ hybridization, polymerase chain reaction, and next-generation sequencing to identify a novel therapeutic target for the prevalent triple-negative subtype of breast carcinoma. Furthermore, a large-scale multicenter study is essential for a better understanding of the nature and prevalence of various types of breast carcinoma in Nigeria.
Conclusion
This study found that breast carcinoma was most prevalent among Nigerian woman in their fifties and that 42.5% of the invasive breast carcinomas had triple-negative immunohistochemical phenotypes similar to the findings among African Americans. Breast cancer is now categorized as a disease of public health importance. Early diagnosis through coordinated breast screening and government support will help mitigate the death toll of breast cancer patients in Nigeria and other African countries.
Disclosure
The authors’ contributions to this paper were as follows: RMW: conception and design of the work, data acquisition, data analysis, manuscript preparation, critical appraisal, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. UBE: manuscript reviewing and editing, literature review, editing, critical appraisal. AOS: literature review and critical reviewing, editing. AEA: design, interpretation of data, drafting and revising, final approval. NAI: literature review and critical reviewing, editing. AAA: literature review and critical reviewing, editing. AAT: statistical analysis.The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgments
We thank Mr. Fowotade, the head scientist of our medical laboratory, and other scientists: Mr. Olutunde, Mr. Balogun Musbau, Mrs. Akanbiola Iyadunni, and Mr. Anafi Hammed for their invaluable help during the processing and staining of tissues for this study.
References
- 1. Ferlay J, Bray F, Pisani P, Parkin DM. Cancer incidence and mortality worldwide: IARC Press Cancer Base 2013;11.
- 2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209-249.
- 3. Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 2013 Mar;132(5):1133-1145.
- 4. Huo D, Ikpatt F, Khramtsov A, Dangou J-M, Nanda R, Dignam J, et al. Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J Clin Oncol 2009 Sep;27(27):4515-4521.
- 5. Chu KC, Anderson WF. Rates for breast cancer characteristics by estrogen and progesterone receptor status in the major racial/ethnic groups. Breast Cancer Res Treat 2002 Jun;74(3):199-211.
- 6. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina breast cancer study. Jama 2006 Jun 7;295(21):2492-2502.
- 7. Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology 2008 Jan;52(1):108-118.
- 8. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19(5):403-410.
- 9. Sinn HP, Kreipe H. A brief overview of the WHO classification of breast tumors, 4th edition, focusing on issues and updates from the 3rd edition. Breast Care (Basel) 2013 May;8(2):149-154.
- 10. Zhu H, Doğan BE. American joint committee on cancer's staging system for breast cancer, Eighth edition: summary for clinicians. Eur J Breast Health 2021 Jun 24;17(3):234-238.
- 11. Fmcpath AA, Fwacs OO, Fmcog SR. Breast cancer trend in a Nigerian population: an analysis of cancer registry data. Life Sci 2012;2(3):29-33.
- 12. Adeniji KA, Bello JM, Durowade KA. Rising pattern of breast cancer in young women. East Afr Med J 2015;92(6):279-283
- 13. Nggada HA, Gali BM, Bakari AA, Yawe-Terna EH, Tahir MB, Apari E, et al. The spectrum of female breast diseases among the Nigerian population in the Sahel Climatic zone. J Med Med Sci 2011;2(10):1157-1161.
- 14. Jedy-Agba EE, Curado MP, Oga E, Samaila MO, Ezeome ER, Obiorah C, et al. The role of hospital-based cancer registries in low and middle income countries-the Nigerian case study. Cancer Epidemiol 2012 Oct;36(5):430-435.
- 15. Awodele O, Adeyomoye AA, Awodele DF, Fayankinnu VB, Dolapo DC. Cancer distribution pattern in south-western Nigeria. Tanzan J Health Res 2011;13(2):125-131.
- 16. Ebughe GA, Ugare GU, Nnoli MA, Bassey IA, Nwagbara VJ, Udosen JE, et al. Histological type and tumour grade in Nigerian breast cancer: relationship to menarche, family history of breast cancer, parity, age at first birth, and age at menopause. IOSR J Dent Med Sci 2013;7(5):58-63.
- 17. Ohene-Yeboah M, Adjei E. Breast cancer in Kumasi, Ghana. Ghana Med J 2012 Mar;46(1):8-13.
- 18. Omar S, Khaled H, Gaafar R, Zekry AR, Eissa S, el-Khatib O. Breast cancer in Egypt: a review of disease presentation and detection strategies. East Mediterr Health J 2003 May;9(3):448-463.
- 19. Saeed IE, Weng HY, Mohamed KH, Mohammed SI. Cancer incidence in Khartoum, Sudan: first results from the cancer registry, 2009-2010. Cancer Med 2014 Aug;3(4):1075-1084.
- 20. Alshammari FD, Hussain GA, Alawad GM, Alshammary M, Alrashdi AG, Alrashedi SA, et al. Epidemiology indicators of cancer in North Saudi Arabia; a population-based survey. Int J Biomed Res 2015;6(9):674-678.
- 21. Youlden DR, Cramb SM, Yip CH, Baade PD. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol Med 2014 Jun;11(2):101-115.
- 22. Bezić J, Tomić S, Kardum G. Minimal breast cancer in split region of Croatia on the eve of the national mammographic screening program. Breast J 2009;15(4):429-431.
- 23. Makanjuola SB, Ayodele SD, Javid FA, Obafunwa JO, Oludara MA, Popoola AO. Breast cancer receptor status assessment and clinicopathological association in Nigerian women: a retrospective analysis. Journal of Cancer Research & Therapy 2014;2(8):122-127.
- 24. Agbo PS, Khalid A, Oboirien M. Clinical presentation, prevalence and management of breast cancer in Sokoto, Nigeria. J Womens Health Care 2014;3(2):1-5.
- 25. Adisa CA, Eleweke N, Alfred AA, Campbell MJ, Sharma R, Nseyo O, et al. Biology of breast cancer in Nigerian women: a pilot study. Ann Afr Med 2012;11(3):169-175.
- 26. Awadelkarim KD, Arizzi C, Elamin EO, Hamad HM, De Blasio P, Mekki SO, et al. Pathological, clinical and prognostic characteristics of breast cancer in Central Sudan versus Northern Italy: implications for breast cancer in Africa. Histopathology 2008 Mar;52(4):445-456.
- 27. Sando Z, Fouogue JT, Kemfang JD, Fouelifack FY. A nationwide study of breast cancer histopathology in Cameroon (Central Africa). J Cytol Histol 2018;9(2):1-4.
- 28. Der EM, Gyasi RK, Tettey Y, Bayor TM, Newman L. Triple-negative breast cancer among ghanaian women seen at Korle-Bu teaching hospital. Trends in Pathology and Microbiology 2015 Mar 31;4(1):1-5.
- 29. Mbonde MP, Amir H, Schwartz-Albiez R, Akslen LA, Kitinya JN. Expression of estrogen and progesterone receptors in carcinomas of the female breast in Tanzania. Oncol Rep 2000;7(2):277-283.
- 30. Maalej M, Hentati D, Messai T, Kochbati L, El May A, Mrad K, et al. Breast cancer in Tunisia in 2004: a comparative clinical and epidemiological study. Bull Cancer 2008 Feb;95(2):E5-E9.
- 31. Ahmad Z, Khurshid A, Qureshi A, Idress R, Asghar N, Kayani N. Breast carcinoma grading, estimation of tumor size, axillary lymph node status, staging, and nottingham prognostic index scoring on mastectomy specimens. Indian J Pathol Microbiol 2009;52(4):477-481.
- 32. Agarwal G, Ramakant P, Forgach ER, Rendón JC, Chaparro JM, Basurto CS, et al. Breast cancer care in developing countries. World J Surg 2009 Oct;33(10):2069-2076.
- 33. Oluogun WA, Adedokun KA, Oyenike MA, Adeyeba OA. Histological classification, grading, staging, and prognostic indexing of female breast cancer in an African population: a 10-year retrospective study. Int J Health Sci (Qassim) 2019;13(4):3-9.
- 34. Ibrahim IM, Iliyasu Y, Mohammed AZ. Histopathological review of breast tumors in Kano, Northern Nigeria. Sub-Saharan Afr J Med 2015;2(1):47-51.
- 35. Roy I, Othieno E. Microscopic study and receptor profile of 45 cases. Arch Pathol Lab Med 2011;135(1):194-198.
- 36. Ukah CO, Emegoakor C, Anyiam DC, Onyiaorah IV, Onwukamuche ME, Egwuonwu OA, et al. The immunohistochemical profile of breast cancer in indigenous women of Southeast Nigeria. Ann Med Health Sci Res 2017;7:83-87.
- 37. Adebamowo CA, Famooto A, Ogundiran TO, Aniagwu T, Nkwodimmah C, Akang EE. Immunohistochemical and molecular subtypes of breast cancer in Nigeria. Breast Cancer Res Treat 2008 Jul;110(1):183-188.
- 38. Gukas ID, Jennings BA, Mandong BM, Igun GO, Girling AC, Manasseh AN, et al. Clinicopathological features and molecular markers of breast cancer in Jos, Nigeria. West Afr J Med 2005;24(3):209-213.
- 39. Usman A, Iliyasu Y, Atanda AT. Molecular subtyping of carcinoma of the female breast in a tertiary teaching hospital in Northern Nigeria. Ann Trop Pathol 2019;10(1):20-26.
- 40. Mahmoud MM. Breast cancer in Kirkuk city, Hormone receptors status (estrogen and progesterone) and Her-2/neu and their correlation with other pathologic prognostic variables. Diyala Journal of Medicine 2014;6(1):1-4.
- 41. Blamey RW, Hornmark-Stenstam B, Ball G, Blichert-Toft M, Cataliotti L, Fourquet A, et al. ONCOPOOL–a European database for 16,944 cases of breast cancer. Eur J Cancer 2010 Jan 1;46(1):56-71.
- 42. Popoola AO, Ibrahim NA, Omodele FO, Oludara MA, Adebowale SA, Igwilo AI. Pattern of spread of breast cancer among patients attending cancer unit of Lagos State University Teaching Hospital. Asian Journal of Medical Sciences 2012 Jun 25;4(3):89-94.
- 43. Shukla A, Jain SC, Swarnkar M. Correlation of axillary lymph nodes involvement and Nottingham prognostic index with various histopathologic prognostic factors in invasive breast carcinoma. International Surgery Journal 2019 Mar 26;6(4):1187-1193.
- 44. Adeniji KA, Huo D, Khramtsov A, Zhang C, Olopade OI. Molecular profiles of breast cancer in Ilorin, Nigeria. J Clin Oncol 2010;28(15):1602.
- 45. Tanimowo MO, Abudu EK, Udo IA, Abdulkareem FB. Histopathological and immunohistochemical characteristics of breast carcinomas in Uyo, Subtropical Region of Africa. Med J Zambia 2019;46(2):100-108.
- 46. Agbo SP, Oboirien M. Risk factors for breast cancer in Sokoto, Nigeria. Merit Res J Med Med Sci 2016;4(11):465-471.
- 47. Kinsella MD, Nassar A, Siddiqui MT, Cohen C. Estrogen receptor (ER), progesterone receptor (PR), and HER2 expression pre- and post- neoadjuvant chemotherapy in primary breast carcinoma: a single institutional experience. Int J Clin Exp Pathol 2012;5(6):530-536.
- 48. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al; American Society of Clinical Oncology; College of American Pathologists. American society of clinical oncology/College of american pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007 Jan;25(1):118-145.
- 49. O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina breast cancer study. Clin Cancer Res 2010 Dec 15;16(24):6100-6110.
- 50. Senegal AT, Haj-Mukhtar NS, Elhaj AM, Bedri S, Kantelhardt EJ, Mohamedani AA. Immunohistochemistry defined subtypes of breast cancer in 678 Sudanese and Eritrean women; in hospital-based case series. BMC Cancer 2017;17(804):1-9.
- 51. Al-Asadi JN, Al-Mayah SM. Three-year survival of women with breast cancer in Basrah, Iraq. Oman Med J 2020 Jul;35(4):e147.
- 52. Eziagu UB, Ndukwe CO, Kudamnya I, Peter AI, Igiri AO. Immunohistochemical survey of invasive ductal carcinoma of the breast, using ER, PR, HER2 and KI-67 biomarkers, in Uyo, Nigeria. Ibom Med J 2022;15(3):223-235.
- 53. Yadav R, Singh S, Marwah N, Kataria K, Aggarwal G, Sen R. Immunohistochemical detection of axillary lymph node micrometastases in breast cancer patients: increasing the accuracy of detection and decreasing labor intensive serial sectioning. Indian J Cancer 2014;51(3):267-271.
- 54. Orang E, Marzony ET, Afsharfard A. Predictive role of tumor size in breast cancer with axillary lymph node involvement - can size of primary tumor be used to omit an unnecessary axillary lymph node dissection? Asian Pac J Cancer Prev 2013;14(2):717-722.