| |
Abstract
Objective: This study aimed to determine the prevalence and assess antimicrobial susceptibility of extended- spectrum β-lactamase-producing Escherichia coli isolated from clinical specimens of patients at hospitals in Khartoum State, Sudan.
Methods: During April to August 2011, a total of 232 E. coli isolates were collected from various clinical specimens of patients. Isolates were identified, tested for antimicrobial susceptibility and screened for ESBL production as per standard methods. The double-disk diffusion method was used to confirm ESBL production using antimicrobial disks of ceftazidime (30 μg), cefotaxime (30 μg), with or without clavulanic acid (10 μg). A zone difference of >5 mm between disks was considered indicative of ESBL production.
Results: Out of 232 E. coli isolates, 70 (30.2%) were found to be positive for ESBL by the applied phenotypic methods. ESBL-producing isolates yielded high resistance rates for trimethoprim-sulfamethoxazole (98.6%), tetracycline (88.6%), nalidixic acid (81.4%) and ciprofloxacin (81.4%). The highest antimicrobial activities of ESBL-producing isolates were observed for amikacin (95.7%), followed by tobramicin (74.3%) and nitrofurantoin (68.6%). Resistance to quinolones, aminoglycosides, trimethoprim-sulfamethoxazole, tetracycline, nitrofurantoin and chloramphenicol was higher in ESBL than non-ESBL isolates (p<0.05). The frequency of ESBL-producing isolates varied among hospitals (18.2% to 45.1%), although a high prevalence was recorded as 45.1% at Khartoum Teaching Hospital. Wound specimens were the most common source of ESBL-producing isolates. The proportion of ESBL-producing E. coli did not differ significantly between adults and children (31% vs. 27%).
Conclusion: The prevalence of ESBL-producing E. coli detected in this study is of great concern, which requires sound infection control measures including antimicrobial management and detection of ESBL-producing isolates.
Keywords: E. coli; Extended- spectrum β-lactamases; Susceptibility; Detection; Sudan.
Introdcution
Extended-spectrum β-lactamases (ESBL) are enzymes produced by many Gram-negative bacteria which have ability to change the susceptibility of different antimicrobial agents.1,2 The ESBL are plasmid-mediated enzymes with the capability to hydrolyze and inactivate broad spectrum of β-Lactam antimicrobials, including third-generation cephalosporins, penicillins and aztreonam; but are inhibited by clavulanic acid.2-4 The two most common plasmid mediated β-Lactamases are the TEM-1 and SHV-1 family mainly expressed in Escherichia coli and Klebsiella pneumoniae, respectively; that confer resistance to antimicrobials.3 ESBL-producing organisms are often also able to reduce the susceptibility of other non-β-lactamase antimicrobial classes, such as aminoglycosides, fluoroquinolones, trimethoprim-sulfamethoxazole, tetracyclines and nitrofurantoin; thus, leaving a limited range of therapeutic agents.5
The growing frequency of ESBL-producing bacteria in clinical settings is causing treatment failure and greater hospital costs due to infections caused by this bacterium.6,7 The presence of ESBL in many E. coli strains are of serious concern, since these organisms are the most common cause of different human infections.4,8 ESBL are becoming a great challenge and an increasing problem for hospitals worldwide.4,9-11
The Clinical and Laboratory Standards Institute (CLSI) recommends the detection of ESBL in Gram-negative bacteria including E. coli by recognizing their decreased susceptibility to the third generation cephalosporins such as ceftazidime, cefotaxime and ceftriaxone.5,10 Once an ESBL is suspected, it should be confirmed by standardized methods.10 The determination of inhibition by clavulanic acid is a common criteria used in all phenotypic methods for the detection of ESBL.4,10 Several methods have been developed to detect the presence of ESBL including double-disk synergy test (DDST) and double-disk diffusion test (DDDT), using cefotaxime and ceftazidime disks with or without clavulanic acid.1 The prevalence of ESBL among pathogenic bacteria varies geographically and in hospital settings, and is rapidly changing over time.8 The frequency of ESBL in Europe is higher than in the USA but lower than in Asia and South America.1,5 In African countries like Sudan, there is generally a lack of comprehensive data with regards to ESBL-producing E. coli.1
The present study was conducted to determine the prevalence and assess the antimicrobial susceptibility patterns of ESBL-producing E. coli as well as non-ESBL producing E. coli recovered from patients at different hospitals in Khartoum State, Sudan, using phenotypic laboratory methods to detect the presence of ESBL-producing isolates.
Methods
This prospective study was carried out during the period of April to August 2011. E. coli isolates were collected from clinical specimens of patients at different educational hospitals in Khartoum State, Sudan. The participating hospitals were: Khartoum Teaching Hospital, Khartoum North Educational Hospital, National Health Laboratory, Omdurman Teaching Hospital, Soba University Hospital, and Turkish Teaching Hospital.
Various clinical specimens were received for routine investigations in each microbiology laboratory of the participating hospitals and were processed for isolation and identification of significant pathogens following conventional procedures.12
Specimen from urine, stool, semen and other body fluids were received into a sterile plastic container and were processed immediately for detection of pathogenic E. coli. However, the specimen of blood were extracted under aseptic conditions and transferred immediately into sterile bottles containing brain heart infusion broth. Specimens isolated from ears, wounds and the vagina were taken as swabs, then placed on transport media and were analyzed as soon as possible.
Isolation of E. coli from specimens of urine, stool, sputum and any other body fluid was done by culturing directly onto MacConkey agar plates (Oxoid, Basingstoke England), using sterile nichrome wire calibrated loop. The isolation of E. coli from clinical specimens of the throat, ears, vaginal and wound swabs was done by inoculating directly onto MacConkey agar plates by streaking the swabs onto a small area of the plate. Then the sterile loop was used for cross-streaking to spread the inoculum over the surface of the plate to obtain single colonies. All cultured plates were incubated aerobically for 24 hours at 37°C and were examined for countable colonies. Each single significant growth of E. coli isolates was included in this study. Culture plates which yielded more than two organisms per specimen were excluded from the study.
E. coli isolates were identified on the basis of cultural characteristics, gram stains, indole production and conventional biochemical tests,13 then confirmed by API 20E identification system (biomerieux Marcy-I’Etoile, France).
Antimicrobial susceptibility testing of E. coli isolates was performed on Mueller-Hinton agar plate (Oxoid, Basingstoke England) by the Kirby-Bauer disk diffusion method following the CLSI recommendations.14 Isolates were tested for their susceptibility against 15 antimicrobial agents including; amikacin (30 μg), amoxicillin (10 μg), amoxicillin-clavulanic acid (30 μg), ceftazidime (30 μg), ceftriaxone (30 μg), cefuroxime (30 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), gentamicin (10 μg), nalidixic acid (30 μg), nitrofurantoin (50 μg), ofloxacin (5 μg), tetracycline (30 μg), tobramicin (10 μg) and trimethoprim-sulfamethoxazole (25 μg), (Oxoid, England). Standardized inoculum conforming to 0.5 McFarland standard turbidity of each isolate was inoculated on two Mueller-Hinton agar plates using a sterile cotton swab by streaking the swab over the entire sterile agar surface 3 times. Then onto each plate, 8 to 9 antimicrobial disks were placed at the recommended distance from each other. All plates were aerobically incubated at 37ºC for 18 hours before the zone sizes were recorded. E. coli ATCC 25922 was used as control strains and was tested each time when susceptibility testing was performed. Test results were only validated in the cases where inhibition zone diameters of the control strains were within performance range in accordance with the CLSI guidelines.14 ESBL detection was done on all E. coli isolates which were screened for ESBL production by the DDST, then confirmed by the double-disk diffusion test (DDDT) as recommended by the CLSI.14
The double-disk synergy test was carried out immediately along with susceptibility testing of each isolates by placing a susceptibility disk of amoxicillin-clavulanic acid at the center of the plate, and disks containing ceftazidime and cefotaxime were placed 30 mm (center to center) from the amoxicillin-clavulanic acid disk. After aerobic incubation at 37ºC for 18 hours, a clear extension of the edge of the inhibition zone of cephalosporin towards amoxicillin-clavulanic acid disk was interpreted as positive for ESBL production.15
The DDDT test was used to confirm that strains were positive by DDST. Standardized inoculum conforming to 0.5 McFarland standards of suspected ESBL-producing isolates were inoculated as previously described. Then, 4 disks containing ceftazidime (30 μg), cefotaxime (30 μg) with and without clavulanic acid (10 μg) were placed at the recommended distance from each other on the plate. The plates were incubated at 37ºC for 18 hours. An increase in the zone diameter greater than or equal to 5 mm for both ceftazidime and cefotaxime tested in combination with clavulanic acid vs. its zone diameter when tested alone, confirmed the presence of an ESBL-producing organism. E. coli strains ATCC 25922 were used as negative controls and Klebsiella pneumoniae ATCC 700603 were used as positive controls.
Statistical analysis was done using the collected data, which were then analyzed using the Statistical Package for Social Sciences (SPSS; Version10.0). The proportions were compared using the Chi-square test. A p-value <0.05 was considered statistically significant.
This was a laboratory based study which had no direct involvement with the concerned patients. All databases which included, specimen sources and patient information such as sex, age and setting, were carefully recorded from laboratory request forms.
Results
A total of 232 E. coli isolates were recovered from patients’ clinical specimens of urine (n=151), wounds (n=51), ear swabs (n=8), high-vaginal swabs (n=7), blood (n=5), miscellaneous body fluids (n=4), seminal fluids (n=4), and from stool (n=2), at different hospitals in Khartoum State, Sudan. Out of the 232 E. coli isolates tested, 70 (30.2%) were found to be ESBL-producers by both applied phenotypic methods. The majority of ESBL-producing isolates were recovered from specimens of urine (n=37) and wound pus (n=28), with decreasing isolation rates from ear swabs (n=2), high-vaginal swabs (n=1), stool (n=1), and miscellaneous body fluids (n=1); while none were found in seminal fluids or in blood specimens. (Fig. 1)
As presented in Fig. 1, the most common source of ESBL-producing isolates were wound specimens 28/51 (55%). The proportion of ESBL-producing E. coli did not differ significantly between adults (31%; 56/181) and children (27%; 14/51).
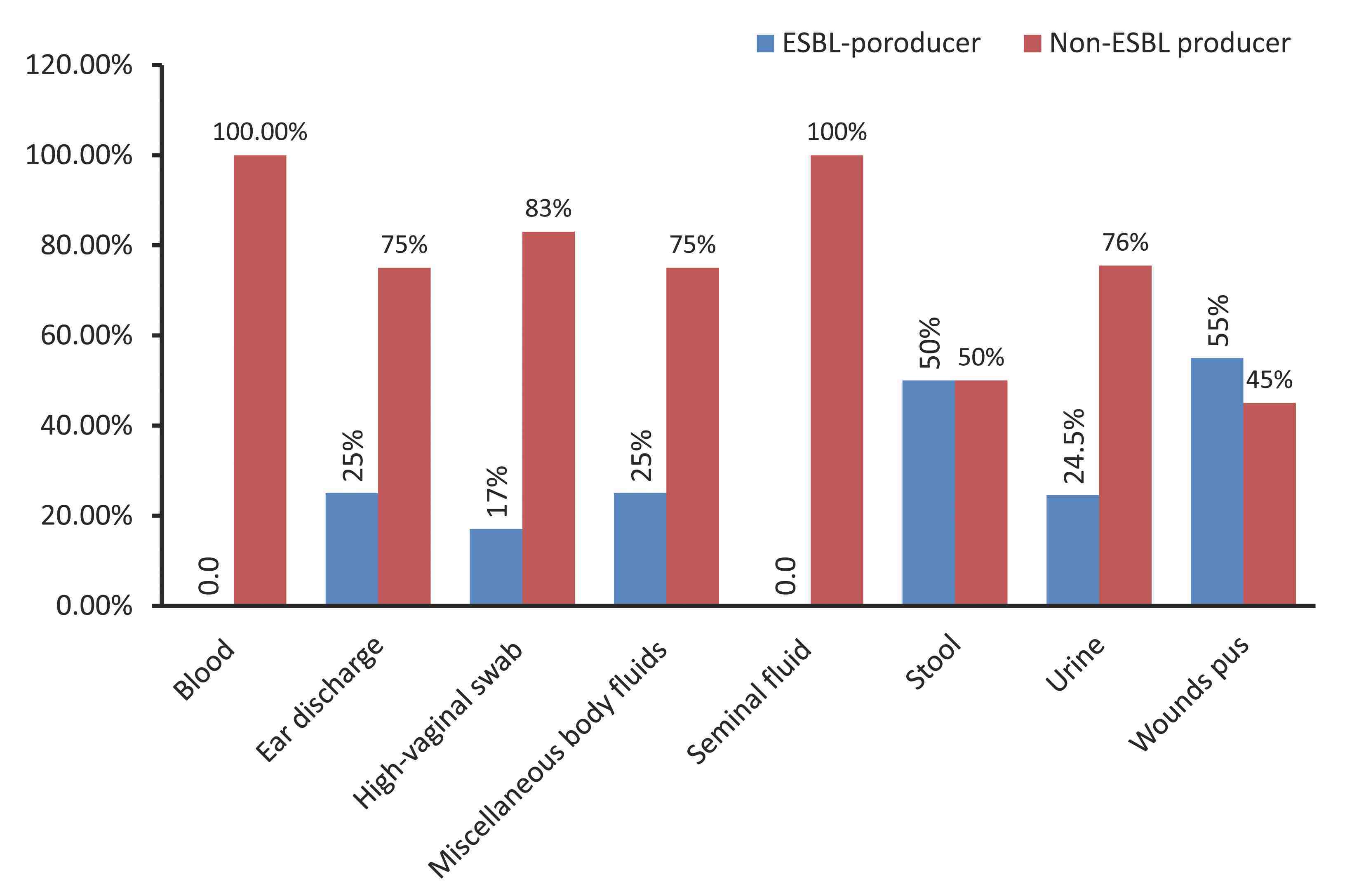
Figure 1: Frequency of ESBL-producing Escherichia coli isolates among various clinical specimens collected from hospitals in Khartoum State, Sudan.
The results of antimicrobial susceptibility of E. coli isolates are shown in Table 1. Among the ESBL-producing E. coli, high resistance rates were observed for trimethoprim-sulfamethoxazole (98.6%), tetracycline (88.6%), ciprofloxacin (81.4%), nalidixic acid (81.4%), ofloxacin (80%), and amoxicillin-clavulanic acid (61.4%). The highest antimicrobial activities of ESBL-producing E. coli were observed with amikacin (95.7%), followed by tobramicin (74.3%), nitrofurantoin (68.6%) chloramphenicol (64.5%), and gentamicin (51.4%). ESBL-producing E. coli isolates were significantly more resistant to trimethoprim-sulfamethoxazole, tetracycline, nalidixic acid, ciprofloxacin, ofloxacin, gentamicin, nitrofurantoin, amoxicillin-clavulanic acid, tobramicin and chloramphenicol compared to non-ESBL producing isolates (p<0.05). (Table 1)
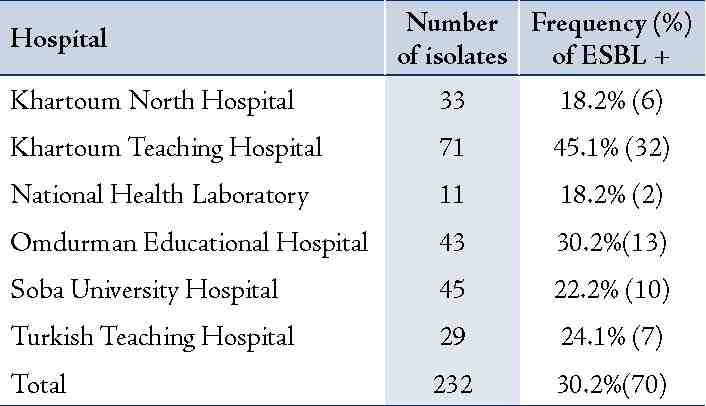
Table 2 summarizes the frequency of ESBL-producing E. coli isolates among different educational hospitals in Khartoum State of Sudan. The highest rates of ESBL-producing E. coli were recovered from Khartoum Hospital (45.1%; 32/71), followed by Omdurman Hospital (30.2%; 13/43), Turkish Hospital (24.1%; 7/29), and Soba Hospital (22.2%; 10/45). While the lowest rates were observed in specimen isolated from Khartoum North Hospital (18.2%; 6/33) and from the National Health Laboratory (18.2%; 2/11).
Table 1: Antimicrobial resistance patterns of ESBL and non-ESBL producing Escherichia coli (%) recovered from patients at hospitals in Khartoum State, Sudan.
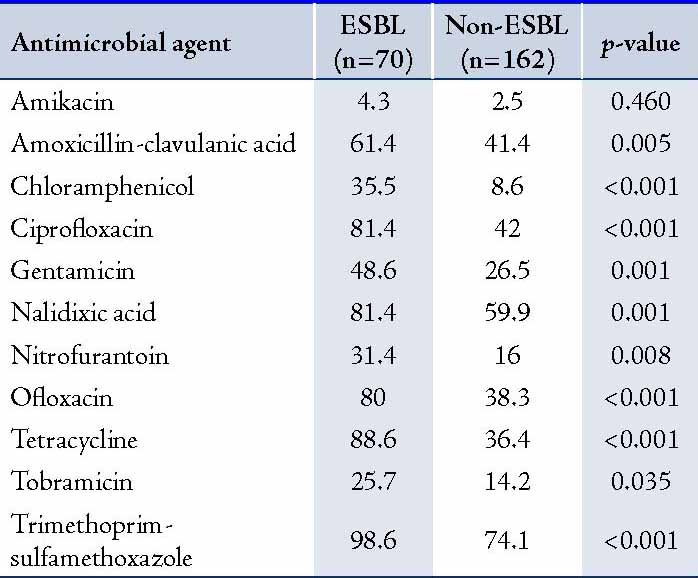
Table 2: Distribution of extended-spectrum β-lactamase-producing Escherichia coli isolated from different hospitals in Khartoum State, Sudan.
Discussion
The present study demonstrated the presence of ESBL-mediated resistance in E. coli isolated from clinical specimens of patients in Sudan hospitals. The prevalence of ESBL-producing E. coli was found to be 30.2%. This figure is low compared to the figure reported in a study carried out in Khartoum State hospitals by Mekki et al.16 who recorded ESBL production among E. coli and Klebsiella species isolates as 53%. But, the current study findings are similar to the 28.2% reported in Tanzania,7 but higher than the 6.5% reported in Saudi Arabia,17 but much lower than the 60.9% observed in Egypt,18 and 78% in Pakistan.9 In addition, the observed prevalence of 30.2% in the current study is much higher compared to those reported in Europe, USA and Canada.1,5 Overall, these findings indicate that the prevalence of bacteria producing-ESBL varies worldwide.
ESBL detection is not routinely carried out in many microbiology laboratories of hospitals in developing countries,19 as well in Sudan. The emergence of ESBL-producing strains create a need for laboratory testing methods for detection of these enzymes among bacterial pathogens.8,10 In the present study, ESBL-producers were detected by DDST and the phenotypic DDDT confirmatory method. These tests were yielded an equal accuracy in the determination of ESBL production. These methods had been previously documented as effective tests for detection of ESBL-producers by other authors.6,8-10 Moreover, these both tests are available and simple to apply routinely along with antimicrobial susceptibility test in our hospitals.
In this study, high resistance rates among ESBL-producing E. coli to first line antimicrobial therapy such as trimethoprim-sulfamethoxazole, tetracycline, nalidixic acid ciprofloxacin and ofloxacin were observed. Similar findings have been reported in developing countries,2,9,11,17,19 as well as in developed countries.5,8 Significantly high rates of resistance to such commonly used oral antimicrobials have been previously described making these agents clinically ineffective for empirical treatment of infection caused by ESBL-producing strains.17,19-21 In the study, ESBL-producing isolates exhibited significantly higher resistant rates to non-β-lactamase antimicrobials agents including fluoroquinolones aminoglycosides, tetracyclines and trimethoprim-sulfamethoxazole, compared to non-ESBL producing isolates. The possible explanation for this observation may in fact be that ESBLs are encoded on plasmids and can be mobile and therefore, easily transmissible as resistance gene elements for other antimicrobials from one organism to another.4,8,18 Al-Yaqoubi and Elhag described that quinolone resistance among E. coli has been reported worldwide and believed it to be due to the acquisition of qnr gene that protects DNA from binding to gyrase and topoisomerase.20 This has been reported to occur more frequently among ESBL producing strains.
In the present study, wound exudates were found to be the most common source of ESBL-producing E. coli (55%). This is in agreement with another study conducted in India by Rudresh and Nagarathnamma,22 where 70% of ESBL-producing isolates were obtained from exudates. Recently, Idowu et al.11 described several factors that may have highly contributed to the occurrence of ESBL-producing isolates in wound infections such as; infection of wounds by microorganisms, which is most often associated with prolonged hospital stay in spite of persistent treatment with antibiotics in different combinations; the attendant risk of acquisition of multiple resistant organisms from medical devices; and hospital environment.
In our study, the occurrence of ESBL-producing E. coli among urine specimens is of great concern, since E. coli is the main causative agent of urinary tract infections; consequently, there is wide spread use of antimicrobial agents due to such infections. ESBL are becoming an increasing problem for hospitals, and the extent of the problem varies greatly from one hospital to another.6,9,10 This was also true in the present study, which revealed that the prevalence of ESBL-producing E. coli isolates varied among the participating hospitals (18.2% to 45.1%); in addition, the results indicated that the highest rate of ESBL-producing E. coli were from specimen isolated at Khartoum hospital (45.1%). Likewise, other authors have reported similar high levels in Tanzania and Egypt.19,21 Nathisuwan et al.4 explained that the major risk factors for colonization or infection with ESBL-producing organisms are: long-term antibiotic exposure; prolonged intensive care unit stay; nursing home residency; severe illness; residence in an institution with frequent use of ceftazidime and other third-generation cephalosporin; and instrumentation or catheterization. Therefore, great focus should be directed towards infection control practices in hospital units in order to prevent the spread of ESBL strains from one patient to another through the following means; ensuring healthcare professionals practice hand hygiene, cleaning medical equipment; and preventing colonization of the environment.1
In the present study, there was no significant difference observed in ESBL-producing isolates among adults compared to isolates from children (31% vs. 27%). A previous study by Moyo et al.19 reported significantly higher ESBL production in isolates from children rather than adults. Likewise, several studies have also investigated the occurrence of ESBL-producing isolates in relation to patient age groups.6,23,24
Conclusion
In summary, the prevalence of ESBL-producing E. coli isolates detected in this study is of great concern, which requires sound infection control measures including antimicrobial management to avoid the risk of therapeutic failure and routine laboratory detection of ESBL-producing isolates in order to decrease their spreading.
Acknowledgements
The authors express gratitude to all staff at the Departments of Microbiology Laboratories of participating hospitals namely: Khartoum, Soba, Omdurman, Khartoum North, National Health Laboratory and Turkish hospitals for help and kind support. The authors reported no conflict of interest and no funding was received for this work.
References
1. Dhillon RH, Clark J. ESBLs: A Clear and Present Danger? Crit Care Res Pract 2012;2012:625170. Published online 6 Jun 2011.
2. Al-Muharrmi Z, Rafay A, Balkhair A, Jabri AA. Antibiotic combination as empirical therapy for extended spectrum Beta-lactamase. Oman Med J 2008 Apr;23(2):78-81.
3. Paterson DL. Recommendation for treatment of severe infections caused by Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs). Clin Microbiol Infect 2000 Sep;6(9):460-463.
4. Nathisuwan S, Burgess DS, Lewis JS II. Extended-spectrum beta-lactamases: epidemiology, detection, and treatment. Pharmacotherapy 2001 Aug;21(8):920-928.
5. Winokur PL, Canton R, Casellas JM, Legakis N. Variations in the prevalence of strains expressing an extended-spectrum beta-lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific region. Clin Infect Dis 2001 May;32(Suppl 2):S94-S103.
6. Tschudin-Sutter S, Frei R, Battegay M, Hoesli I, Widmer AF. Extended spectrum β-lactamase-producing Escherichia coli in neonatal care unit. Emerg Infect Dis 2010 Nov;16(11):1758-1760.
7. Ndugulile F, Jureen R, Harthug S, Urassa W, Langeland N. Extended spectrum β-lactamases among Gram-negative bacteria of nosocomial origin from an intensive care unit of a tertiary health facility in Tanzania. BMC Infect Dis 2005;5:86.
8. Bradford PA. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev 2001 Oct;14(4):933-951.
9. Hussain M, Hasan F, Shah AA, Hameed A, Jung M, Rayamajhi N, et al. Prevalence of class A and AmpC β-lactamases in clinical Escherichia coli isolates from Pakistan Institute of Medical Science, Islamabad, Pakistan. Jpn J Infect Dis 2011;64(3):249-252.
10. Florijn A, Nijssen S, Schmitz FJ, Verhoef J, Fluit AC. Comparison of E test and double disk diffusion test for detection of extended spectrum beta-lactamases. Eur J Clin Microbiol Infect Dis 2002 Mar;21(3):241-243.
11. Idowu OJ, Onipede AO, Orimolade AE, Akinyoola LA, Babalola GO. Extended-spectrum Beta-lactamase Orthopedic Wound Infections in Nigeria. J Glob Infect Dis 2011 Jul;3(3):211-215.
12. Thomson RB Jr, Miller JM. Specimen collection, transport, and processing: bacteriology. In: Murray PR, Baron EJ, Pfaller MA, Tenover, FC and Yolken RH, ed. Manual of Clinical Microbiology. 8th ed. Washington, DC: American Society for Microbiology, 2003. p. 286-330.
13. Farmer JJ III. Enterobacteriaceae: Introduction and Identification. In: Murray PR, Baron EJ, Pfaller MA, Tenover, FC and Yolken RH, ed. Manual of Clinical Microbiology. 8th ed. Washington, DC: American Society for Microbiology, 2003. p. 636-653.
14. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility tests: Informational supplement 21 ed. CLSI document M100-S21. CLSI: Wayne, Pa; 2011.
15. Jarlier V, Nicolas MH, Fournier G, Philippon A. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis 1988 Jul-Aug;10(4):867-878.
16. Mekki AH, Hassan AN, Elsayed DE. Extended spectrum beta lactamases among multi drug resistant Escherichia coli and Klebsiella species causing urinary tract infections in Khartoum. Journal of Bacteriology Research 2010;2(3):18-21.
17. Kader AA, Kumar AK. Prevalence of extended spectrum β-lactamase among multidrug resistant gram-negative isolates from a general hospital in Saudi Arabia. Saudi Med J 2004 May;25(5):570-574.
18. Mohamed Al-Agamy MH, El-Din Ashour MS, Wiegand I. First description of CTX-M beta-lactamase-producing clinical Escherichia coli isolates from Egypt. Int J Antimicrob Agents 2006 Jun;27(6):545-548.
19. Moyo SJ, Aboud S, Kasubi M, Lyamuya EF, Maselle SY. Antimicrobial resistance among producers and non-producers of extended spectrum beta-lactamases in urinary isolates at a tertiary Hospital in Tanzania. BMC Res Notes 2010;3:348.
20. Al-Yaqoubi M, Elhag K. Susceptibilities of common bacterial isolates from oman to old and new antibiotics. Oman Med J 2008 Jul;23(3):173-178.
21. Zaki MelS. Extended spectrum β-lactamases among gram-negative bacteria from an Egyptian pediatric hospital: a two-year experience. J Infect Dev Ctries 2007;1(3):269-274.
22. Rudresh SM, Nagarathnamma T. Extended spectrum β-lactamase producing Enterobacteriaceae & antibiotic co-resistance. Indian J Med Res 2011 Jan;133:116-118.
23. Ho PL, Wong RC, Chow KH, Yip K, Wong SS, Que TL. CTX-M type beta-lactamases among fecal Escherichia coli and Klebsiella pneumoniae isolates in non-hospitalized children and adults. J Microbiol Immunol Infect 2008 Oct;41(5):428-432.
24. Tandé D, Jallot N, Bougoudogo F, Montagnon T, Gouriou S, Sizun J. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in a Malian orphanage. Emerg Infect Dis 2009 Mar;15(3):472-474.
|
|