Group B streptococcus (GBS), also known as Streptococcus agalactiae, is a gram-positive coccus with β-hemolysis. For a long time, GBS has been recognized as one of the most common causes of neonatal infections but it’s a rare encounter in older children. GBS is known to cause bacteremia, skin and soft tissue infection, and neonatal sepsis but is uncommon to cause infective endocarditis.1
Here, we report a case of recurrent GBS infective endocarditis in 11 years old child. The child had a complex cardiac surgical history with possible prosthetic mitral valve infective endocarditis complicated by heart failure. As he was not fit for surgical intervention, the recurrence of infection was suppressed by one-year-long antibiotic therapy.
This case illustrates that S. agalactiae can infect children beyond the neonatal period where it can contribute to conditions such as infective endocarditis. The prolonged antimicrobial suppressive therapy is an alternative option when surgery is not possible even in complicated cases of infective endocarditis.
Case Report
An 11-year-old Omani boy presented to the emergency department with two days’ history of high-grade fever, headache, and nausea. A known patient of Loeys-Dietz syndrome with ascending aortopathy and mitral valve regurgitation, he had previously undergone mitral valve repair followed by valve-sparing aortic root replacement.
On examination, the child was febrile (38.9 °C), not in respiratory distress, hemodynamically stable, well-perfused, and had a regular pulse. Systolic murmur grade III was noted at upper sternum. Blood investigations showed leukocytosis of 19.9 × 109 /L, neutrophilia of 17.5 × 109 /L, and C-reactive protein of 341 mg/L. Urine microscopy, urea, and electrolyte levels were normal. Apical 4 chamber 2D echocardiogram showed echo dense mass of 17 × 7 mm attached to the posterior mitral valve leaflet [Figure 1]. Blood culture collected on admission flagged positive the next day for gram-positive cocci in chains, identified as S. agalactiae using an automated machine (Phoenix) and it was sensitive to Penicillin (MIC ≤ 0.1 μg/mL). On admission, he was started empirically on ceftriaxone and vancomycin. On the third day of admission, based on the GBS blood culture report, the antibiotics were changed to penicillin G (400 000 units/kg/day q6h IV) and gentamicin (2 mg/kg q8h IV).
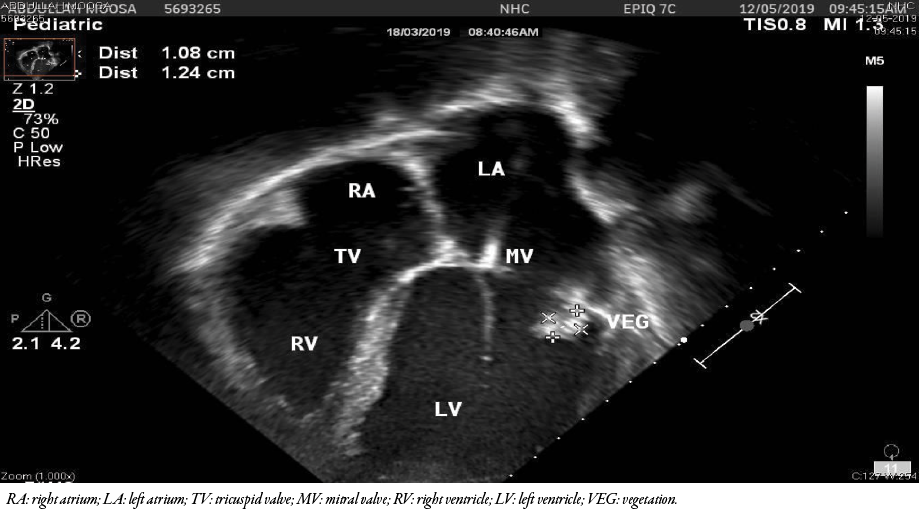 Figure 1: Transthoracic 2D echocardiography at presentation showing echo-dense mass attached to the posterior mitral valve leaflet.
Figure 1: Transthoracic 2D echocardiography at presentation showing echo-dense mass attached to the posterior mitral valve leaflet.
On the second day of admission, the patient had delirium; a computed tomography scan of the brain yielded normal results. Blood cultures taken on days two, five, and 10 of admission came negative for GBS due to its sensitivity to ceftriaxone. Though the fever spikes continued for > 10 days, their frequency and grade gradually diminished. Transesophageal echocardiography on day 10 suggested an irregular dense mass at mitral valve measuring 15 × 8 mm, moderate mitral valve regurgitation, mild aortic valve regurgitation, and aortic valve leaflets that were thin with no vegetation, and no echo evidence of an abscess. The patient became afebrile after two weeks of treatment with penicillin G and gentamicin and was discharged as per the convenience of the family.
Outpatient therapy continued on ceftriaxone (50 mg/kg q12h IV). Repeated transthoracic echocardiography on week four showed the same echo-dense mass as before and moderate mitral valve regurgitation. The child remained afebrile with recovery of inflammatory markers and negative blood culture. After completing six weeks of antibiotics, repeat transthoracic echocardiography showed the same echogenic mass which appeared denser and smaller than at presentation, now measuring 7.2 mm, suggestive calcification of the vegetation [Figure 2].
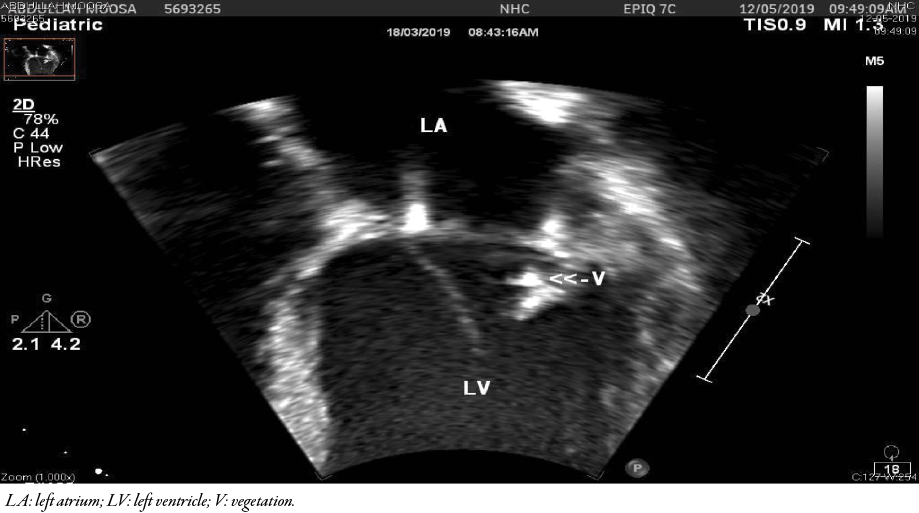 Figure 2: Transthoracic echocardiography after six weeks of antibiotics showed the same echogenic mass which appeared denser and smaller than before.
Figure 2: Transthoracic echocardiography after six weeks of antibiotics showed the same echogenic mass which appeared denser and smaller than before.
After six months, he presented again with a picture of clinical meningitis and arrhythmia. Blood culture again grew GBS which was sensitive to penicillin. A lumbar puncture was not performed due to the patient’s instability. Transthoracic echocardiography showed only fibrotic lesion at mitral valve because of the previous surgery and cardiac magnetic resonance imaging showed no identifiable vegetation (but vegetation embedded within surgical material could not be excluded). Again, he was treated for infective endocarditis with the same antibiotic regimen as before; repeated transthoracic echocardiogram at the end of six weeks showed no vegetation. Serotyping of S. agalactiae came as Ib. He was prescribed oral suppressive penicillin for one year without recurrence during follow-up. Unfortunately, the patient passed away six months after due to heart failure exacerbated by chest infection. The heart failure could be related to infective endocarditis as the patient had progressed from mild to moderate mitral regurgitation during the period of nfective endocarditis.
Discussion
We reported a rare occurrence of S. agalactiae infective endocarditis in an 11-year-old child with recurrence in six months. Despite clearance of infection with prolonged suppressive therapy, the patient passed away due to heart failure secondary to severe inoperable mitral regurgitation. Cases of GBS-caused infective endocarditis are reported either in the neonatal period or adulthood.2–4 Infective endocarditis due to GBS infection in adolescents is exceedingly rare and we could find no previous report in the English literature.
The diagnosis of infective endocarditis in the current case was made in accordance with the modified Duke’s criteria.5 Duke proposed one major criteria (vegetation at echocardiography) and three minor criteria high-grade fever > 38 °C, pre-existing cardiac disease, positive blood culture not fitting major criteria as only one set of blood culture which still could be major criteria if the patient received antibiotic before collecting other sets of blood culture.5
GBS endocarditis is more common with native valve; prosthetic valve infective endocarditis tends to have poorer outcome.6,7 The most frequently involved in GBS infective endocarditis is mitral valve as in our patient.7 Serotypes of GBS are currently divided into 10, based on type specific capsular antigens Ia, Ib, II, III, IV, V, VI, VII, VIII, and IX.8 Our patient had Ib serotype isolate. Generally with GBS, there is no evidence of any association between the serotypes and the virulence, specific underlying conditions, or outcome of infection.9 In neonates, serotype Ia is the second-most prevalent one in early onset GBS, whereas serotype Ib is the second most prevalent in late onset-GBS; in North America, serotypes Ia, Ib, II, III, and V have been linked mostly to invasive neonatal disease.10,11 However, most reports of invasive GBS infections in adults have not defined the serotype including the largest case series involving 30 adults with infective endocarditis.4
The complications of GBS infective endocarditis are embolization (particularly in the brain), mycotic aneurysms, massive organ infarction, heart failure, and significant mortality.6,12,13 The child described in this report had clinical meningitis in both his presentations though central nervous system imaging was not supportive and obtaining cerebrospinal fluid sample was contraindicated. The patient’s mitral valve disease got worse because of resurgent infection and resulted in severe regurgitation and death after two years from his initial presentation due to heart failure. Cardiac surgery is usually indicated in such cases. However, our patient was deemed inoperable because of his original disease and previous surgeries.
Conclusion
GBS can cause severe and fatal infective endocarditis in older children as in neonates. A larger multicenter registry is required for a better understanding of epidemiology and pathology, and to plan better interventions including prophylaxis and vaccination.
Disclosure
The authors declared no conflicts of interest. Consent was obtained from the patient's mother.
references
- 1. Raabe VN, Shane AL, Group B. Streptococcus (Streptococcus agalactiae). Microbiol Spectr 2019 Mar;7(2).
- 2. Streptococcus agalactiae - an overview | ScienceDirect Topics. [cited 2019 May 18]. Available from: https://www.sciencedirect.com/topics/medicine-and-dentistry/streptococcus-agalactiae.
- 3. Graux E, Hites M, Martiny D, Maillart E, Delforge M, Melin P, et al. Invasive group B Streptococcus among non-pregnant adults in Brussels-Capital Region, 2005-2019. Eur J Clin Microbiol Infect Dis 2021 Mar;40(3):515-523.
- 4. Navarro-Torné A, Curcio D, Moïsi JC, Jodar L. Burden of invasive group B Streptococcus disease in non-pregnant adults: a systematic review and meta-analysis. PLoS One 2021 Sep;16(9):e0258030.
- 5. Baltimore RS, Gewitz M, Baddour LM, Beerman LB, Jackson MA, Lockhart PB, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young and the Council on Cardiovascular and Stroke Nursing. Infective endocarditis in childhood: 2015 update: a scientific statement from the American heart association. Circulation 2015 Oct;132(15):1487-1515.
- 6. Abdelghany M, Schenfeld L. Group B streptococcal infective endocarditis. J Infect Public Health 2014 May-Jun;7(3):237-239.
- 7. Abid L, Charfeddine S, Kammoun S. Isolated Streptococcus agalactiae tricuspid endocarditis in elderly patient without known predisposing factors: case report and review of the literature. Journal of the Saudi Heart Association 2016 Apr 1;28(2):119-123.
- 8. Centers for Disease Control and Prevention. Streptococcus laboratory: streptococcus agalactiae | CDC. 2019 [cited 2019 May 18]. Available from: https://www.cdc.gov/streplab/groupb-strep/index.html.
- 9. Berg S, Trollfors B, Lagergård T, Zackrisson G, Claesson BA. Serotypes and clinical manifestations of group B streptococcal infections in western Sweden. Clin Microbiol Infect 2000 Jan;6(1):9-13.
- 10. Zhu Y, Wu J, Zheng X, Liu D, Xu L, Chen D, et al. Etiological serotype and genotype distributions and clinical characteristics of group B streptococcus-inducing invasive disease among infants in South China. BMC Pediatr 2020 Apr;20(1):146.
- 11. Lo CW, Liu HC, Lee CC, Lin CL, Chen CL, Jeng MJ, et al. Serotype distribution and clinical correlation of Streptococcus agalactiae causing invasive disease in infants and children in Taiwan. Journal of Microbiology, Immunology and Infection 2019 Aug 1;52(4):578-584.
- 12. Achilli P, Guttadauro A, Bonfanti P, Terragni S, Fumagalli L, Cioffi U, et al. Streptococcus agalactiae infective endocarditis complicated by multiple mycotic hepatic aneurysms and massive splenic infarction: a case report. BMC Gastroenterol 2017 Dec;17(1):170.
- 13. Fujita H, Nakamura I, Tsukimori A, Sato A, Ohkusu K, Matsumoto T. Severe infective endocarditis in a healthy adult due to Streptococcus agalactiae. Int J Infect Dis 2015 Sep;38:43-45.