Infective endocarditis (IE) in the peripartum period has a low incidence and hence our knowledge regarding its presentation and management is limited. The traditional risk factors associated with IE include structural heart diseases, the presence of prosthetic valves/intravascular devices, chronic hemodialysis, and continuous intravenous drug use with the latter being the major risk factor in recent years.1 Suppressed immunity during pregnancy, the postpartum period, and invasive procedures performed during delivery may result in mucosal barrier disruption and systemic infection by opportunistic organisms.2 We hereby report a case of native valve right-sided IE in a healthy female who had a recent vaginal delivery of a healthy baby one month prior to presentation.
Case report
A 20-year-old postpartum female (para 1 living 1) was admitted with high-grade intermittent fever up to 38.9 οC associated with chills for 20 days, insidious onset cough with mucoid expectoration and gradually progressive shortness of breath from modified Medical Research Council grade II to IV over a period of 14 days along with streaky hemoptysis around two to three episodes per day for four days. On further inquiry, she revealed a history of cough associated with mucoid expectoration in her ninth month of pregnancy. She received treatment from a local hospital with relief of symptoms. She delivered a healthy baby 15 days later via normal vaginal delivery with no intervention during pregnancy and no peripartum complications. She had presented to our hospital around 25 days after the delivery with symptom onset of around 4–5 days post-delivery. She belonged to lower class of social economic strata as per Indian guidelines. There was no history of intravenous drug use or any history suggestive of rheumatic heart disease.
At presentation, she had systolic blood pressure of 122 mmHg and diastolic blood pressure of 68 mmHg with a pulse of 120 beats/min and a respiratory rate of 30 breaths/min with saturation of 98% at room air and temperature of 38.9 οC. On examination, she had pallor with grade II holosystolic murmur at left lower sternal border. Laboratory investigations revealed hemoglobin of 7.7 g/dL, total leucocyte count of 13 × 109/L, and a platelet count of 200 × 109/L. Chest computed tomography (CT) showed bilateral peripheral nodules and consolidation with cavitary changes with subpleural location suggestive of septic emboli [Figure 1]. A suspicion of infective valve endocarditis with septic emboli was kept and transthoracic 2D echocardiography revealed 3 × 1 cm vegetation on anterior leaflet of tricuspid valve [Figure 2]. Blood culture grew methicillin-resistant Staphylococcus aureus. She fulfilled two major clinical criteria (vegetation on 2D echocardiography and blood culture growth of typical organism) and two minor criteria (fever and vascular phenomenon) according to the modified Duke’s criteria for IE. The patient was started on intravenous injection of vancomycin 1 g every 12 hours. During her hospital stay, she had sudden onset hemoptysis requiring elective intubation. She was immediately resuscitated with intravenous fluids and transfusion of packed red blood cells. Repeat chest CT showed cavitating nodules in both lungs with new consolidative changes noted in right lower lobe. In view of the new consolidation and fever, intravenous injection meropenem 1 g was added every 8 hours. She was subsequently extubated within 48 hours. The patient remained afebrile with no fresh episode of hemoptysis. All repeat sets of blood cultures were sterile. She continued on antibiotics for six weeks. A written and well-informed consent was taken from the patient. She was discharged in stable condition with systolic blood pressure of 118 mmHg and diastolic blood pressure of 80 mmHg, and a pulse of 84 bpm. 2D echocardiography at the time of discharge showed residual vegetation of around 1 × 0.5 cm in size with no ventricular dysfunction or valve regurgitation. She has been advised three monthly echocardiography for one year and six monthly thereafter. Since there is no residual valve dysfunction, there seems to be no contraindication for future pregnancy.
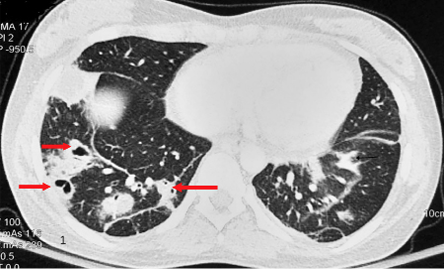 Figure 1: Contrast-enhanced CT of chest showing bilateral segmental and peripheral areas of consolidation with cavitary changes with subpleural location of few on right side suggestive of septic emboli (arrows).
Figure 1: Contrast-enhanced CT of chest showing bilateral segmental and peripheral areas of consolidation with cavitary changes with subpleural location of few on right side suggestive of septic emboli (arrows).
 Figure 2: Apical four chamber view of 2D echocardiography of heart showing vegetation of 3 × 1 cm on tricuspid valve with no regurgitation (arrow).
Figure 2: Apical four chamber view of 2D echocardiography of heart showing vegetation of 3 × 1 cm on tricuspid valve with no regurgitation (arrow).
Discussion
IE is a serious and fatal complication of heart valves. There has been a gradual increase in the annual incidence of IE from 5–7 cases per 100 000 person-years (1970–2000)1 to around 15 cases per 100 000 population in the USA in 2011.3 IE in peripartum period has low incidence and hence our knowledge regarding its presentation and management is limited. Overall reported incidence in pregnancy is 1 in 100 000 pregnancies,2 but is responsible for high maternal (22.1%) and fetal mortality (14.7%).4 Most commonly identified risk factors in pregnancy are intravenous drug use (14.4%), congenital heart disease (12.2%), and rheumatic heart disease (11%).5 Most common pathogenic organisms are streptococcus species (39%) followed by staphylococcus (25.6%). Left-sided cases are more common than right-sided and are more likely due to streptococcal infection than right side, which are due to staphylococcus infection.5
Suppressed immunity during pregnancy, the postpartum period, and invasive procedures performed during delivery may result in mucosal barrier disruption and systemic infection by opportunistic organisms. In our case since the actual symptom onset was prior to pregnancy, which was overlooked and later exacerbated post-delivery, risk factor precipitating the infection could not be determined. Case-control studies on IE have revealed larger vegetations (> 1–2 cm in size) to be associated with increased morbidity and mortality.6 Although the risk of pulmonary emboli is high with tricuspid vegetation and increases morbidity, the response to antibiotic therapy is generally favorable with right-sided lesions.4 In a recent literature review; maternal mortality was 11.5% in pregnant cases and 10.5% in postpartum cases. Mortality was lower in right-sided cases compared to left-sided cases (6.1% vs. 14.3%).5
In the setting of right-sided native valve endocarditis, medical therapy with culture sensitivity guided intravenous antibiotics constitutes the mainstay of management while surgical interventions are often deferred unless there is; (i) right-sided heart failure due to severe tricuspid regurgitation, (ii) persistent bacteremia for > 7 days or fungemia refractory to medical therapy, (iii) large vegetations of > 20 mm, (iv) recurrent multiple pulmonary emboli with or without right side heart failure, or (v) abscess formation.7 Multi-disciplinary approach involving early assessment by the infectious disease expert, obstetrician, cardiologists, and cardiovascular surgeons will result in better prognosis of peripartum IE patients.
Conclusion
IE is rare in pregnancy and the postpartum period, but has high maternal and fetal mortality. Early diagnosis and aggressive management targeting specific organisms and a multi-disciplinary team approach can result in a good outcome.
Disclosure
The authors declared no conflicts of interest. A written consent was obtained from the patient.
references
- 1. Tleyjeh IM, Steckelberg JM, Murad HS, Anavekar NS, Ghomrawi HM, Mirzoyev Z, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. JAMA 2005 Jun;293(24):3022-3028.
- 2. Montoya ME, Karnath BM, Ahmad M. Endocarditis during pregnancy. South Med J 2003 Nov;96(11):1156-1157.
- 3. Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015 May;65(19):2070-2076.
- 4. Campuzano K, Roqué H, Bolnick A, Leo MV, Campbell WA. Bacterial endocarditis complicating pregnancy: case report and systematic review of the literature. Arch Gynecol Obstet 2003 Oct;268(4):251-255.
- 5. Kebed KY, Bishu K, Al Adham RI, Baddour LM, Connolly HM, Sohail MR, et al. Pregnancy and postpartum infective endocarditis: a systematic review. Mayo Clin Proc 2014 Aug;89(8):1143-1152.
- 6. Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992 Oct;117(7):560-566.
- 7. Shmueli H, Thomas F, Flint N, Setia G, Janjic A, Siegel RJ. Right-sided infective endocarditis 2020: challenges and updates in diagnosis and treatment. J Am Heart Assoc 2020 Aug;9(15):e017293.