Congenital uterine anomaly (CUA) is a type of female genital malformation that results from abnormal formation, fusion, and resorption of the Müllerian duct during the early process of development in the fetus.1 Malformation can occur at any stage of Müllerian development and the resultant uterine anomalies can be of diverse types and shapes.2,3
Most women with CUA are asymptomatic. Differing diagnostic techniques and classification criteria have led to wide variations in the estimated prevalence of CUA.4 A widely used classification of CUA is the one developed by the American Fertility Society [Table 1].5 The most commonly reported CUA type is arcuate uterus, although considered by some clinicians as an anatomical variant.4 Among the other types of CUA, septate uterine anomaly is associated with poor pregnancy outcomes, while bicornuate uteri and unicornuate uteri are linked to high rates of infertility.6,7 A meta-analysis of studies on CUA, infertility and pregnancy outcomes found higher rates of infertility and spontaneous abortion in CUA patients.8
Table 1: Common types of congenital uterine anomaly as per the American Fertility Society.
|
Arcuate
|
Cavity indentation of endometrium at the fundus.
|
|
Bicornuate
|
Uterine duplication due to partial failure of Müllerian ducts fusion.
|
|
Unicornuate
|
Failure of one of the Müllerian ducts to form leading to one horned uterus.
|
|
Septate
|
Failure of the resorption of the septum after Müllerian ducts fusion leading to duplicate uterine cavities in case of complete failure.
|
Studying the prevalence and types of CUA in relation to infertility is crucial because the role of these anomalies in causing infertility is still unclear.9,10
Fallopian tube anomalies, both genetic and acquired, are often reported in patients with CUA.10 Indeed, acquired anomalies of the fallopian tube are considered a significant cause of primary and secondary infertility,11 although there is no clear estimate of their prevalence in general and infertile female populations; mention of fallopian tube anomalies in the literature is limited mostly to case reports.12
The prevalence of CUA in Oman remains unknown. This is an important research gap because of CUA’s reported role in miscarriages and infertility.13 In addition, the causes of infertility in Omani women are understood insufficiently. Apart from baseline blood tests, the most common diagnostic tool for female infertility is hysterosalpingography (HSG). It is routinely used to investigate the fallopian tubes and the uterine cavity, because their abnormalities are known to be responsible for half the cases of infertility and subfertility.14–16 Our study aimed to narrow the research gap by retrospectively estimating the prevalence and types of CUAs and tubal blockage in infertile Omani women, based on patient records at a teaching hospital in Oman.
Methods
This retrospective study was conducted by reviewing the HSG of outpatients and inpatients who had been investigated, treated, and followed-up at a teaching hospital in Muscat. All patients who underwent this investigational procedure in the five-year period from January 2013 to December 2018 were included in the study. The HSG radiographic reports were collected and analyzed for the presence and type of CUA and tubal blockage. Descriptive statistical analyses of the demographic data and radiological findings were performed using the SPSS Statistics (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.). Categorical data were expressed as frequencies and percentages. The chi-square test assessed possible associations between the variables. P-value < 0.05 was considered statistically significant with a CI of 95%.
Ethical approval was granted by the medical research ethics committee at the College of Medicine and Health Sciences at Sultan Qaboos University, Muscat, Oman (Ref. SQU-EC/169/19, MREC#1975).
Results
The retrieved data pertaining to N = 912 Omani women in the age group 19–48 years (mean = 31.6±5.7) was included in the study. The majority of the patients (552; 60.5%) were aged ≥ 30 years. Most patients (508; 55.7%) had been investigated for secondary infertility and 404 (44.3%) for primary infertility. Patients < 30 years had been diagnosed mainly with primary infertility while those aged ≥ 30 years were more likely to have had secondary infertility (p < 0.001) [Figure 1].
Table 2 gives the details of the HSG findings in the patients included in this study. Based on HSG, 27 (3.0%) patients were found to have CUA. The commonest type of CUA found by HSG was the arcuate uterus [Figure 2]. No significant correlation was found between the presence of CUA and the type of infertility.
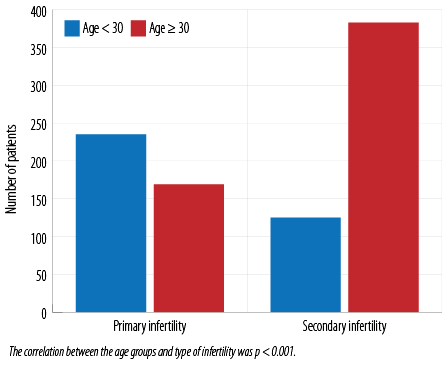 Figure 1: Age groups of patients investigated by hysterosalpingography for different types of infertility.
Figure 1: Age groups of patients investigated by hysterosalpingography for different types of infertility.
Table 2: Association of the presence of congenital uterine anomalies detected by hysterosalpingogram with age and infertility type.
|
< 30 years
|
Primary
|
229 (25.1)
|
6 (0.7)
|
235 (25.8)
|
|
Secondary
|
123 (13.5)
|
2 (0.2)
|
125 (13.7)
|
|
≥ 30 years
|
Primary
|
167 (18.3)
|
2 (0.2)
|
169 (18.5)
|
|
Secondary
|
366 (40.1)
|
17 (1.9)
|
383 (42.0)
|
CUA: congenital uterine anomalies. All percentages are based on N = 912.
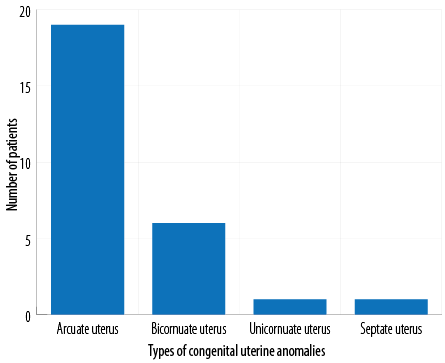 Figure 2: Number of patients with different types of congenital uterine anomalies detected by hysterosalpingography.
Figure 2: Number of patients with different types of congenital uterine anomalies detected by hysterosalpingography.
Fallopian tube blockage was reported in 203 (22.3%) patients. The prevalence of tubal blockage among those investigated for primary infertility was 80 of 203 (39.4%), of whom 123 of 203 (60.6%) were investigated for secondary infertility. Most the patients with fallopian tube block were aged ≥ 30 years (145 of 203; 71.4%). Figures 3a and 3b show the prevalence of fallopian tube block according to infertility type and age groups, respectively.
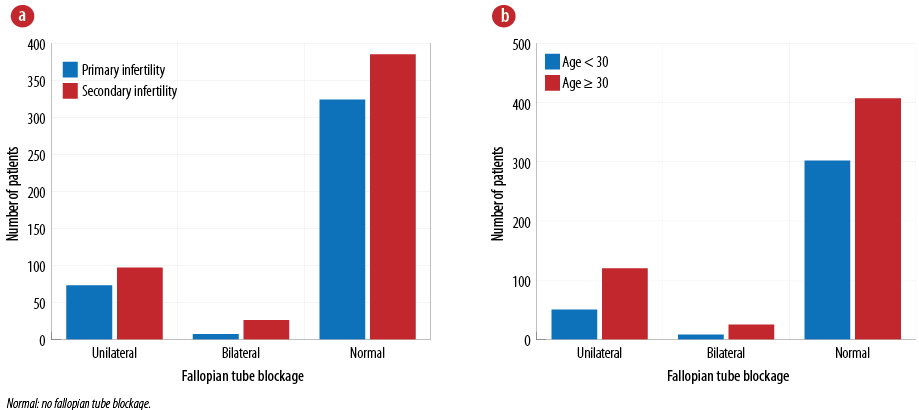 Figure 3: Distribution of fallopian tube blockage among N = 912 patients based on (a) the type of infertility and (b) age group.
Figure 3: Distribution of fallopian tube blockage among N = 912 patients based on (a) the type of infertility and (b) age group.
Eight of the 27 (29.6%) patients diagnosed with CUA were found to have a tubal blockage in the same HSG. Bilateral tubal block was found in two patients; one had an arcuate uterus anomaly and the second had a bicornuate uterus. The remaining six patients had unilateral tubal blockage with arcuate uterus anomaly. All patients with concurrent arcuate uterus and tubal blockage were investigated for secondary infertility. In contrast, only one patient with bicornuate uterus and bilateral tubal blockage was investigated for primary infertility.
In the current cohort, 180 of 912 (19.7%) women suffered pregnancy loss. CUA was reported in seven of these 180 patients (3.9%). There was one case (0.6%) with tubal blockage and arcuate uterus form of CUA [Table 3].
Table 3: Normal uterus and congenital uterine anomalies (CUAs) found in hysterosalpingogram of n = 180 subjects who suffered a loss of pregnancy.
|
< 30 years
|
76 (42.2)
|
1 (0.6)
|
77 (42.8)
|
All percentages are based on n = 180.
Discussion
HSG is a common method to investigate infertility, probes the uterine cavity and the uterine tubes. Even though other modalities such as hysteroscopy, sonohysterogram, and magnetic resonance imaging evaluate the uterine cavity and CUAs more accurately,14,17–21 HSG remains the most popular initial test.14
The prevalence of CUAs varies widely because of different diagnostic procedures and the subjectivity in the criteria used for diagnosis as well as the inconsistencies in interpreting the CUA classification.22–24 The prevalence of CUA in the infertile Omani women in this study was 3.0%. The patients with a history of pregnancy loss formed 3.9%. These levels are consistent with many published studies,23,25,26 though some others reported higher prevalence rates.25 Our results, as do other studies, emphasize the discrepancies in the reported prevalence which could be attributed to some reporters subjectively classifying cases of uteri with minor indentations as ‘normal’ and those with more pronounced indentations as ‘sub-septate uteri’. 9,10,25,27
An arcuate uterus was the most common type of CUA diagnosed in the cohort we studied. This finding goes hand in hand with what has been shown in some publications.23,28 However, Nahum et al,28 found septate uterus to be the most common CUA type in their Saudi Arabian cohort. Considering the ethnic similarities between Saudis and Omanis, this discrepancy could be attributed to adopting different methods of investigation, variable classification systems, and the subjectivity of the reporters from either side.
Another Saudi Arabian study, using methods similar to ours, studied HSG reports of 117 Saudi women with fertility issues.14 The incidence of primary infertility was 41% against 44% in our study. The Saudi women with primary infertility tended to be younger and more prone to anomalies than their counterparts with secondary infertility. Further, 81.2% of the Saudi cohort had fallopian abnormalities and 23% had uterus-related defects.14 A recent study, also from Saudi Arabia, reported uterine malformations in 6.8% of their cohort of 75 patients against our 3%.28 Part of the differences in the findings of these two Saudi studies vis-à-vis ours is perhaps due to their much smaller cohorts—117 and 75, compared to our 912. Differing and conflicting results make it difficult to compare the studies from different parts of the world. An additional skewing factor affecting international comparisons is the likelihood of genetic and cultural heterogeneity between study populations.25,29
Our study suggests that CUA is more common in Omani patients with secondary infertility. Most women with CUA remain asymptomatic, especially those having the types of abnormalities found in our cohort30 in whom the most common CUA type was arcuate uterus. There has been a longstanding debate about the existence and significance of the arcuate uterus, as it is regarded as a minor anomaly by most common classification systems: the European Society of Human Reproduction and Embryology - European Society of Gynecological Endoscopy and the American Society of Reproductive Medicine systems.4 We found seven cases of CUA among 180 who suffered pregnancy loss (3.9%), six of which were arcuate uterus type. Other causes of pregnancy loss should be investigated as well, to clarify whether arcuate uterus anomaly was the major cause of the pregnancy loss reported here. Having said that, interpretation of results related to arcuate uterus relation with reproductive health should be taken with caution as until now there is no robust conclusion regarding the causes of infertility in patients presenting with CUA.25
The effect of different CUA forms on patients’ fertility and reproductive outcomes is debatable. Furthermore, a controversy is existing in the reproductive field regarding the proper management of patients with CUA to improve reproductive outcomes.25 As most published studies do not differentiate between individual types of CUA but present the data collectively, future studies should explore the types of CUA and suggest possible cause-effect relationship between each type and its impact on fertility and overall reproductive health.
Tubal blockage was found in 29.6% of our patients. We also found tubal abnormalities to be more common than uterine anomalies, in agreement with some published studies.28,31 We found only one case with combined tubal blockage and uterine anomalies, similar to the report by Toufig et al.28 More cases with unilateral tubal blockage were reported in our study in agreement with what was reported by some studies.28,32
No significant association was detected between the CUA and Fallopian tube blockage in our cohort, similar to Toufig et al.28 However, we found more cases with CUA and Fallopian tube blockage in patients investigated for secondary infertility in contrast to what is reported by Toufig et al.28 Having more cases with CUA investigated for secondary infertility provide reassurance of the mild effect of the type of CUA detected in the cohort we studied. Many studies reported arcuate uterus as a mild anomaly and considering it as a normal variant is still debatable. More studies are needed to settle this uncertainty.
Furthermore, the relatively higher prevalence of fallopian tube blockage in patients investigated for secondary infertility necessitates further investigation. It is highly recommended to investigate such patients for pelvic inflammatory disease and sexually transmitted diseases, post delivery/abortion.
Among the several limitations of our study is its retrospective nature. Though our cohort was a large one, it was sourced from a single hospital. Another limitation was using HSG, which can only evaluate the interior contour of uterine cavity. Inclusion of patients evaluated for infertility alone is yet another drawback of our study as it may have missed asymptomatic women. Moreover, subjective variations in diagnosis among different radiologists reporting the results could not be avoided.
Conclusion
This study, the first assessment of the prevalence and types of CUA in the Omani population, found CUAs in 3.0% of a cohort of 912 women. The most prevalent CUA type was arcuate uterus. We believe our findings will provide a background for future studies on CUA and female reproductive health. We recommend further investigations on the prevalence and characteristics of CUA in several institutions located in different parts of Oman. Studies that compare the diagnostic efficacy of HSG with other modalities are also suggested. Furthermore, we recommend prospective studies investigating the relationship between women’s reproductive health and different types of CUA.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Moore AB, Flake GP, Swartz CD, Heartwell G, Cousins D, Haseman JK, et al. Association of race, age and body mass index with gross pathology of uterine fibroids. J Reprod Med 2008 Feb;53(2):90-96.
- 2. Saravelos SH, Cocksedge KA, Li TC. Prevalence and diagnosis of congenital uterine anomalies in women with reproductive failure: a critical appraisal. Hum Reprod Update 2008 Sep-Oct;14(5):415-429.
- 3. Propst AM, Hill III JA. Anatomic factors associated with recurrent pregnancy loss. In Seminars in Reproductive Medicine 2000;18(04):341-350.
- 4. Prior M, Richardson A, Asif S, Polanski L, Parris-Larkin M, Chandler J, et al. Outcome of assisted reproduction in women with congenital uterine anomalies: a prospective observational study. Ultrasound Obstet Gynecol 2018 Jan;51(1):110-117.
- 5. Adhesions AD. The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, mullerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49(6):944.
- 6. Grimbizis G, Camus M, Clasen K, Tournaye H, De Munck L, Devroey p. Hysteroscopic septum resection in patients with recurrent abortions or infertility. Hum Reprod 1998 May;13(5):1188-1193.
- 7. Gliozheni O, Gliozheni E. Congenital uterine anomalies: impact on perinatal outcomes. Orion 2021;15(1).
- 8. Venetis CA, Papadopoulos SP, Campo R, Gordts S, Tarlatzis BC, Grimbizis GF. Clinical implications of congenital uterine anomalies: a meta-analysis of comparative studies. Reprod Biomed Online 2014 Dec;29(6):665-683.
- 9. Homer HA, Li TC, Cooke ID. The septate uterus: a review of management and reproductive outcome. Fertil Steril 2000 Jan;73(1):1-14.
- 10. Taylor E, Gomel V. The uterus and fertility. Fertil Steril 2008 Jan;89(1):1-16.
- 11. Briceag I, Costache A, Purcarea VL, Cergan R, Dumitru M, Briceag I, et al. Fallopian tubes–literature review of anatomy and etiology in female infertility. J Med Life 2015 Apr-Jun;8(2):129-131.
- 12. Vaiarelli A, Luk J, Patrizio p. Ectopic pregnancy after IVF in a patient with unilateral agenesis of the fallopian tube and ovary and with endometriosis: search of the literature for these associations. J Assist Reprod Genet 2012 Sep;29(9):901-904.
- 13. Akhtar MA, Saravelos SH, Li TC, Jayaprakasan K; Royal College of Obstetricians and Gynaecologists. Reproductive implications and management of congenital uterine anomalies: scientific impact paper No. 62 November 2019. BJOG 2020 Apr;127(5):e1-e13.
- 14. Al-Turki HA, Gullenpet AH, Syed A, Al-Saif HS, Aldhafery BF. Uterine and tubal abnormalities in infertile Saudi Arabian women: a teaching hospital experience. Saudi J Med Sci 2016 May-Aug;4(2):89-92.
- 15. Brown SE, Coddington CC, Schnorr J, Toner JP, Gibbons W, Oehninger S. Evaluation of outpatient hysteroscopy, saline infusion hysterosonography, and hysterosalpingography in infertile women: a prospective, randomized study. Fertil Steril 2000 Nov;74(5):1029-1034.
- 16. Imaoka I, Wada A, Matsuo M, Yoshida M, Kitagaki H, Sugimura K. MR imaging of disorders associated with female infertility: use in diagnosis, treatment, and management. Radiographics 2003 Nov-Dec;23(6):1401-1421.
- 17. Taşkın EA, Berker B, Özmen B, Sönmezer M, Atabekoğlu C. Comparison of hysterosalpingography and hysteroscopy in the evaluation of the uterine cavity in patients undergoing assisted reproductive techniques. Fertil Steril 2011 Aug;96(2):349-352.e2.
- 18. Zafarani F, Ahmadi F. Evaluation of intrauterine structural pathology by three-dimensional sonohysterography using an extended imaging method. Int J Fertil Steril 2013 Apr;7(1):1-6.
- 19. Omari EA, Varghese T, Kliewer MA. A novel saline infusion sonohysterography-based strain imaging approach for evaluation of uterine abnormalities in vivo: preliminary results. J Ultrasound Med 2012 Apr;31(4):609-615.
- 20. Tur-Kaspa I, Gal M, Hartman M, Hartman J, Hartman A. A prospective evaluation of uterine abnormalities by saline infusion sonohysterography in 1,009 women with infertility or abnormal uterine bleeding. Fertil Steril 2006 Dec;86(6):1731-1735.
- 21. Ma L, Wu G, Wang Y, Zhang Y, Wang J, Li L, et al. Fallopian tubal patency diagnosed by magnetic resonance hysterosalpingography. J Reprod Med 2012 Sep-Oct;57(9-10):435-440.
- 22. Salim R, Regan L, Woelfer B, Backos M, Jurkovic D. A comparative study of the morphology of congenital uterine anomalies in women with and without a history of recurrent first trimester miscarriage. Hum Reprod 2003 Jan;18(1):162-166.
- 23. Grimbizis GF, Camus M, Tarlatzis BC, Bontis JN, Devroey P. Clinical implications of uterine malformations and hysteroscopic treatment results. Hum Reprod Update 2001 Mar-Apr;7(2):161-174.
- 24. Woelfer B, Salim R, Banerjee S, Elson J, Regan L, Jurkovic D. Reproductive outcomes in women with congenital uterine anomalies detected by three-dimensional ultrasound screening. Obstet Gynecol 2001 Dec;98(6):1099-1103.
- 25. Hassan MA, Lavery SA, Trew GH. Congenital uterine anomalies and their impact on fertility. Womens Health (Lond) 2010 May;6(3):443-461.
- 26. Nahum GG. Uterine anomalies. How common are they, and what is their distribution among subtypes? J Reprod Med 1998 Oct;43(10):877-887.
- 27. Kupesic S. Clinical implications of sonographic detection of uterine anomalies for reproductive outcome. Ultrasound Obstet Gynecol 2001 Oct;18(4):387-400.
- 28. Toufig H, Benameur T, Twfieg ME, Omer H, El-Musharaf T. Evaluation of hysterosalpingographic findings among patients presenting with infertility. Saudi J Biol Sci 2020 Nov;27(11):2876-2882.
- 29. Chan YY, Jayaprakasan K, Zamora J, Thornton JG, Raine-Fenning N, Coomarasamy A. The prevalence of congenital uterine anomalies in unselected and high-risk populations: a systematic review. Hum Reprod Update 2011 Nov-Dec;17(6):761-771.
- 30. Pellerito JS, McCarthy SM, Doyle MB, Glickman MG, DeCherney AH. Diagnosis of uterine anomalies: relative accuracy of MR imaging, endovaginal sonography, and hysterosalpingography. Radiology 1992 Jun;183(3):795-800.
- 31. Okafor CO, Okafor CI, Okpala OC, Umeh E. The pattern of hysterosalpingographic findings in women being investigated for infertility in Nnewi, Nigeria. Niger J Clin Pract 2010 Sep;13(3):264-267.
- 32. Aziz MU, Anwar S, Mahmood S. Hysterosalpingographic evaluation of primary and secondary infertility. Pak J Med Sci 2015 Sep-Oct;31(5):1188-1191.