Leiomyoma of the tracheobronchial tree is a rare tumor of the respiratory tract accounting for < 2% of benign lung tumors.1 Symptoms depend on the degree of airway obstruction.1–4 Bronchial leiomyoma has a benign course, and complete local resection of the tumor is therapeutic.
We report a patient with symptomatic endobronchial leiomyoma that was resected employing lung parenchyma preserving techniques. This report stresses the importance of preoperative diagnostic workup for successful lung preservation.
Case report
A previously healthy, 33-year-old man, presented with two years history of cough and breathlessness. He was diagnosed with bronchial asthma and was treated accordingly but showed no improvement. In the last year, he had recurrent chest infections requiring frequent antibiotics.
On examination, he had decreased air entry in the right middle and lower lung zones. Chest computed tomography (CT) scan showed an ovoid shaped endobronchial lesion in the right main bronchus, extending into the trachea causing a total collapse of the right middle and lower lobes [Figure 1].
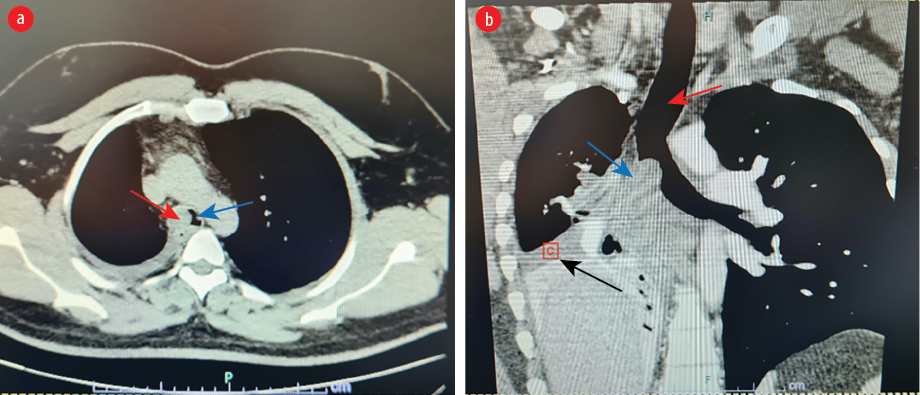 Figure 1: (a) Axial views of chest CT scan showing an ovoid mass (red arrow) obstructing the right main bronchus bulging into the carina and distal trachea (blue arrow). (b) Coronal views showing trachea (red arrow), mass (blue arrow) and collapse of the right middle and lower lobes and mediastinal shift to the right (black arrow).
Figure 1: (a) Axial views of chest CT scan showing an ovoid mass (red arrow) obstructing the right main bronchus bulging into the carina and distal trachea (blue arrow). (b) Coronal views showing trachea (red arrow), mass (blue arrow) and collapse of the right middle and lower lobes and mediastinal shift to the right (black arrow).
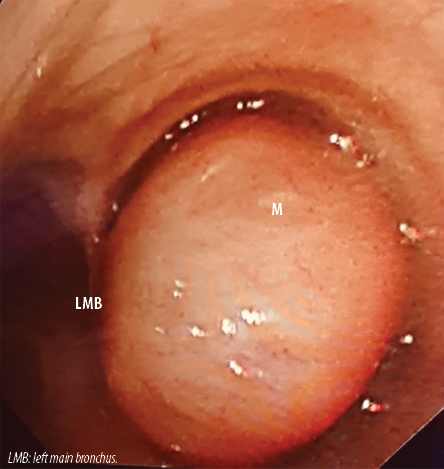 Figure 2: Bronchoscopic view of the mass (M) within the right main bronchus bulging into the trachea resulting in near total occlusion of the right main bronchial lumen. LMB: left main bronchus.
Figure 2: Bronchoscopic view of the mass (M) within the right main bronchus bulging into the trachea resulting in near total occlusion of the right main bronchial lumen. LMB: left main bronchus.
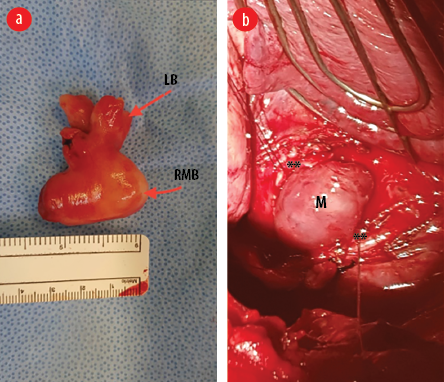 Figure 3: (a) Gross appearance of the mass after surgical removal. The mass has taken the shape of the right main bronchus (RMB) and the lobar bronchus (LB). (b) Intraoperative image showing the right main bronchus open with the edges retracted with stay sutures (**) and the mass (M) appears to be bulging out of the bronchotomy.
Figure 3: (a) Gross appearance of the mass after surgical removal. The mass has taken the shape of the right main bronchus (RMB) and the lobar bronchus (LB). (b) Intraoperative image showing the right main bronchus open with the edges retracted with stay sutures (**) and the mass (M) appears to be bulging out of the bronchotomy.
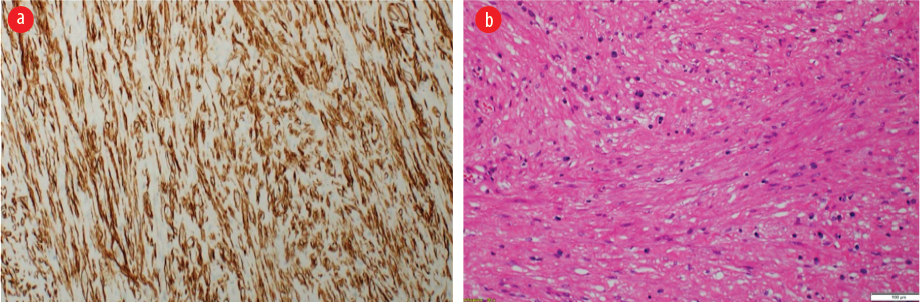 Figure 4: (a) Microscopy of the neoplasm shows a cellular proliferation of intersecting fascicle of bland spindle cells. No nuclear atypia, necrosis, or increased mitoses were identified. (Hematoxylin and eosin stain, magnification = 20 ×). (b) These neoplastic cells are positive for h-Caldesmon immunostain,
Figure 4: (a) Microscopy of the neoplasm shows a cellular proliferation of intersecting fascicle of bland spindle cells. No nuclear atypia, necrosis, or increased mitoses were identified. (Hematoxylin and eosin stain, magnification = 20 ×). (b) These neoplastic cells are positive for h-Caldesmon immunostain,
magnification = 10 ×.
Bronchoscopy confirmed the presence of a smooth, lobulated oval mass bulging into the distal trachea from the right main bronchus. It was impossible to pass the bronchoscope beyond the mass. The mass was pale, non-pulsating, and appeared like an endobronchial carcinoid [Figure 2]. Punch biopsies were taken. However, the mass did not bleed as is usually the case with endobronchial carcinoids. The biopsies revealed polypoid/nodular fragments of tissue lined by benign bronchial epithelial cells. The cores showed spindle cells in the edematous stroma. The spindle cells were positive for desmin, h-Caldesmon, and smooth muscle actin. The mass was diagnosed as endobronchial leiomyoma.
The patient underwent a mini right posterolateral thoracotomy. The middle and lower lobes appeared normal despite repeated infections. However, it remained collapsed despite attempted positive ventilation. Bronchotomy of the right main bronchus extending to the bronchus intermedius was performed. The endobronchial mass bulged out of the bronchotomy [Figure 3a]. It had a wide base that was attached to the bronchus intermedius. We resected the attachment together with a small margin of normal bronchial wall. The bronchial opening was closed with interrupted sutures to ensure that the bronchus was not narrowed. The suture line was reinforced with a parietal pleura flap. The right lung was inflated fully under positive pressure ventilation.
Postoperatively, the patient did well, and the right lung remained fully expanded. The mass was sent for histopathological examination. Macroscopic examination revealed a well-circumscribed nodular mass measuring 4 × 3.5 × 2 cm with a smooth outer surface [Figure 3b]. The cut section showed a homogenous, solid, white-whorled surface. The microscopy showed a well-circumscribed variably cellular spindle cell neoplasm partially lined by respiratory type epithelium. It is composed of fascicles of bland spindle cells having cigar-shaped nuclei and abundant eosinophilic cytoplasm. The stroma shows variable hyalinization with thin and thick-walled blood vessels and scattered mast cells. There is no atypia, increased mitosis, or coagulative necrosis. Immunohistochemical stains were performed, and the neoplastic spindle cells were strongly positive for smooth muscle actin, desmin, and h-Caldesmon [Figure 4]. They were negative for cytokeratin AE1/AE3, CD34, S100, and STAT 6. The final histopathological diagnosis was endo-bronchial leiomyoma.
At one-year follow-up, his symptoms had completely resolved. Imaging showed fully expanded lung with no evidence of recurrence.
Discussion
Pulmonary leiomyoma of 51% occurs in the parenchyma, 33% in the bronchial tree, and only 16% in the trachea. They present in adults with a slight female preponderance and have been reported in patients with Epstein-Barr virus, HIV/AIDS, and cellular immunodeficiency.4,5 Symptoms depend on the site and degree of airway compromise. They can present with cough, wheezing, shortness of breath, and/or chest pain.1–3 Parenchymal disease is asymptomatic and is usually an incidental finding.1
Diagnosis requires imaging, bronchoscopy, and histopathological biopsy. There are no pathognomonic radiological features of leiomyoma. It may show atelectasis or hyperinflation of the lung due to airway obstruction. CT scan has almost 100% sensitivity for detecting the mass but is not specific. The mass shows a homogeneous, smooth, or lobulated appearance with diffuse enhancement and a well-delineated margin. However, such features are shared with leiomyosarcoma, lipoma, and neurogenic tumors.3 Both carcinoid and leiomyoma appear lobulated, central, and may have eccentric calcification.6 Magnetic resonance imaging provides no clear diagnostic advantage over CT.5 Thus, imaging alone is insufficient to differentiate benign from malignant endobronchial pathologies.
Hence, it is important to perform bronchoscopy to visualize the location, shape of the base of the mass, get a biopsy for a definitive histological diagnosis, and plan definitive therapy.1,3 The risks associated with endobronchial biopsy are very low. The mortality risk is almost nil, while the complications are mostly minor and self-limiting and do not exceed 10% in most cases.7
Cases of endobronchial resection of the tumor have been described. Methods used include argon plasma coagulation, Nd:YAG laser, biopsy forceps extraction, electrocautery, and snare removal.2 The tumor is first mechanically removed by forceps or snare, followed by laser ablation of the stump. If the tumor is for ablation only, it can be vaporized with Nd:YAG laser using a flexible bronchoscope.8 These are less invasive techniques and contribute to a faster and less eventful recovery. However, such minimally invasive endoluminal methods are limited to technical and anatomical factors. Polypoid lesions and broad-based lesions are not suitable as complete resection is not possible as they may extend outside the cartilage.2 Fell et al,7 reported tumor growth of these lesions with a doubling in size of an endobronchial lesion within 18 months, potentially predisposing to hemorrhage from neovascularization and making long-term bronchoscopic follow-up of treated lesions imperative.7
Endoluminal resection therapies require repeated sessions and lifetime surveillance and have a high recurrence rate.1 As such, they should be reserved for palliative cases unsuitable for surgery and pediatric cases to delay surgical resection.2
Definitive treatment requires complete excision of the mass to prevent a recurrence.1 Many patients present with advanced lung destruction from recurrent infections requiring major lung resections such as lobectomy or pneumonectomy.2,3 This emphasizes the need for early intervention and tumor removal before airway obstruction results in repeated lung infections and destruction. Every effort should be made to preserve as much lung parenchyma as possible as this is a benign pathology and most patients are young, with a median age of 46 years.1 Lung preserving techniques such as wedge resection, segmentectomy, bronchoplasty, and sleeve resection are important in young patients and those with limited lung reserve. Perioperative and long-term morbidity and mortality have been associated with more extensive lung resection.9 Thus, limiting resection to that needed to treat the pathology is very important.
Conclusion
Endobronchial leiomyoma is a rare benign pathology. Making a definitive preoperative diagnosis is important as similarly appearing pathologies require more aggressive intervention. Limited but complete excision of the mass should always be the goal. Our patient underwent excision of leiomyoma with complete preservation of the lung parenchyma which was only possible because of the definitive diagnosis on preoperative biopsy.
Disclosure
The authors declared no conflicts of interest. Informed consent has been obtained from the patients.
references
- 1. Park JS, Lee M, Kim HK, Choi YS, Kim K, Kim J, et al. Primary leiomyoma of the trachea, bronchus, and pulmonary parenchyma–a single-institutional experience. Eur J Cardiothorac Surg 2012 Jan;41(1):41-45.
- 2. Dmello D, Javed A, Espiritu J, Matuschak GM. Endobronchial leiomyoma: case report and literature review. J Bronchology Interv Pulmonol 2009 Jan;16(1):49-51.
- 3. Cárdenas-García J, Lee-Chang A, Chung V, Shim C, Factor S, Tibb A. Bronchial leiomyoma, a case report and review of literature. Respir Med Case Rep 2014 May;12:59-62.
- 4. Swarnakar R, Sinha S. Endobronchial leiomyoma: a rare and innocent tumour of the bronchial tree. Lung India 2013 Jan;30(1):57-60.
- 5. Saoud M, Patil M, Dhillon SS, Pokharel S, Picone A, Hennon M, et al. Rare airway tumors: an update on current diagnostic and management strategies. J Thorac Dis 2016 Aug;8(8):1922-1934.
- 6. Park JS, Lee M, Kim HK, Choi YS, Kim K, Kim J, et al. Primary leiomyoma of the trachea, bronchus, and pulmonary parenchyma–a single-institutional experience. Eur J Cardiothorac Surg 2012 Jan;41(1):41-45.
- 7. Fell CD, Tremblay A, Michaud GC, Urbanski S. Electrocauterization of an endobronchial leiomyoma. J Bronchol. 2005;12:181-183.
- 8. Jeung MY, Gasser B, Gangi A, Charneau D, Ducroq X, Kessler R, et al. Bronchial carcinoid tumors of the thorax: spectrum of radiologic findings. Radiographics 2002 Mar-Apr;22(2):351-365.
- 10. Ozeki N, Kawaguchi K, Okasaka T, Fukui T, Fukumoto K, Nakamura S, et al. Marginal pulmonary function is associated with poor short- and long-term outcomes in lung cancer surgery. Nagoya J Med Sci 2017 Feb;79(1):37-42.