Primary cardiac tumors are rare with an estimated incidence of approximately 0.3–0.7% in surgery and autopsy reports.1 Approximately 25% of primary cardiac tumors are malignant.2 Angiosarcomas and undifferentiated pleomorphic sarcoma were previously thought to represent the most common primary malignant cardiac tumors. However, analysis of 100 primary cardiac sarcomas using fluorescence in situ hybridization for mouse double minute 2 (MDM2) amplification, as a classification parameter, has suggested that intimal sarcomas are the most common primary cardiac sarcomas (42/100 cases).3
Cardiac intimal sarcomas are frequently misdiagnosed as benign myxoma and thrombus. Cardiac magnetic resonance (CMR) is a non-ionizing imaging modality that can be utilized to diagnose cardiac tumors pre-operatively.4 Herein, we present a 55-year-old man who presented with a history of dizziness and was found to have a right ventricular outflow tract (RVOT) mass on echocardiogram. Further assessment with CMR revealed a well-circumscribed enhancing mobile mass that was protruding into the main pulmonary artery (PA) through the pulmonary valve during the systolic phase resulting in complete obliteration of the main PA. The radiological features were favoring a cardiac myxoma. The patient underwent surgical resection of the mass and the histopathology results confirmed the diagnosis of cardiac intimal sarcoma. The information provided in this article aims to increase the awareness of radiologists regarding this rare entity that can simulate benign lesions. To the best of our knowledge, this is the first case of cardiac intimal sarcoma diagnosed in Oman.
Case report
A 55-year-old man, known to be hypertensive and diabetic, presented to the emergency department with a two-week history of dizziness, which was exacerbated by exercise and relieved by rest. He denied any history of chest pain, fever, cough, or shortness of breath.
Electrocardiogram was done and showed T-wave inversion in the anterior leads. His chest radiograph was unremarkable. Laboratory investigations including troponin, complete blood count, liver function test, and renal function were normal. An echocardiogram was requested and showed a RVOT mass that extended into the main pulmonary trunk, measuring 5 × 2.5 cm. The mass was obstructing the main pulmonary trunk giving a maximal gradient of 30 mmHg. In addition, there were mild concentric left ventricular hypertrophy and a small pericardial effusion. Otherwise, there was no evidence of valvular disease and the ejection fraction was normal (65.0%). In view of the echocardiogram findings, a pulmonary angiography computed tomography (CT) was requested, which confirmed the presence of a RVOT well-defined hypodense mass extending to the main pulmonary trunk. The impression was a pulmonary embolism versus a myxoma with pulmonary embolism favored over a tumor.
A CMR was requested for further characterization of the mass and revealed a well-defined mass arising within the RVOT. The mass was protruding into the main pulmonary trunk during the systole phase and causing almost a complete occlusion of main PA. It was isointense on steady-state free precession images, hyperintense on T2-weighted images, hypointense on T1, and measured 3.6 × 4 × 5.5 cm [Figure 1]. The mass demonstrated an early first-pass perfusion and intense post-contrast enhancement on the delayed images [Figure 2]. The findings on MRI were inkeeping with a neoplasm and confidently excluded thrombus. Differential diagnoses of the mass included myxoma and angiosarcoma. The patient underwent surgical excision of the RVOT mass, and it was sent for histopathological examination.
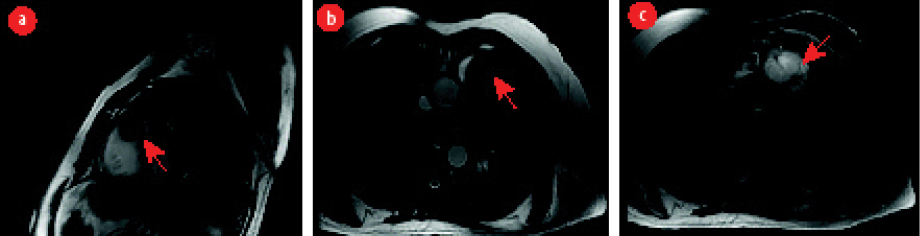 Figure 1: (a, b) Cine steady-state free precession magnetic resonance imaging showing a well-defined isointense mass located within the right ventricular outflow tract (red arrows). (c) The mass is hyperintense in T2-weighted image.
Figure 1: (a, b) Cine steady-state free precession magnetic resonance imaging showing a well-defined isointense mass located within the right ventricular outflow tract (red arrows). (c) The mass is hyperintense in T2-weighted image.
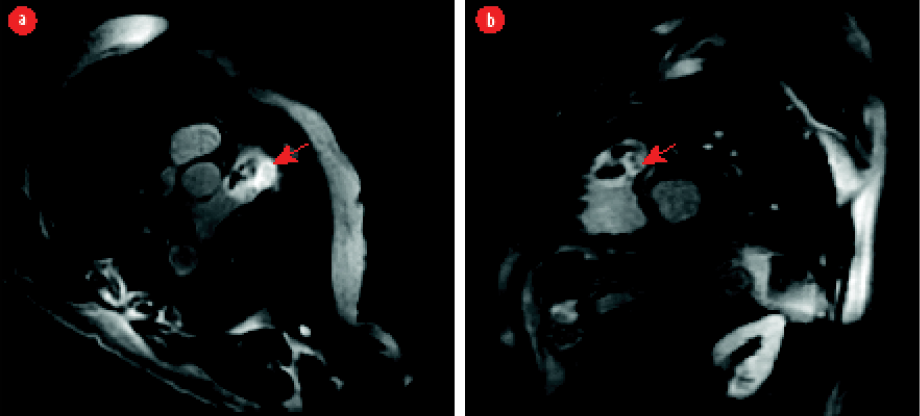 Figure 2: Late gadolinium (a) axial and (b) short axis oblique views showing delayed enhancement of the tumor with areas of non-enhancement representing necrosis (red arrows).
Figure 2: Late gadolinium (a) axial and (b) short axis oblique views showing delayed enhancement of the tumor with areas of non-enhancement representing necrosis (red arrows).
The resected specimen was a circumscribed light brown firm nodule weighing 25.4 g and measuring 5.7 cm in maximum dimension. The cut surface was grey/white with a few yellow-brown areas, focal myxoid areas, and tiny cystic spaces [Figure 3]. Microscopically, the tumor was composed of sheets of malignant cells with hyperchromatic and markedly pleomorphic nuclei. Some of the cells showed clear or valcuolated cytoplasm. The tumor contained many variably sized vascular spaces. Areas of myxoid degeneration, hemorrhage, and necrosis were also seen. The malignant cells were focally positive for MDM2, p16, spinal muscular atrophy, and CD31. Other vascular markers (CD34, factor VIII, D2-40, and FLI-1) were all negative. Epithelial and other muscle markers were also negative. S100 was negative [Figure 4]. The histological findings were consisting of an intimal sarcoma.
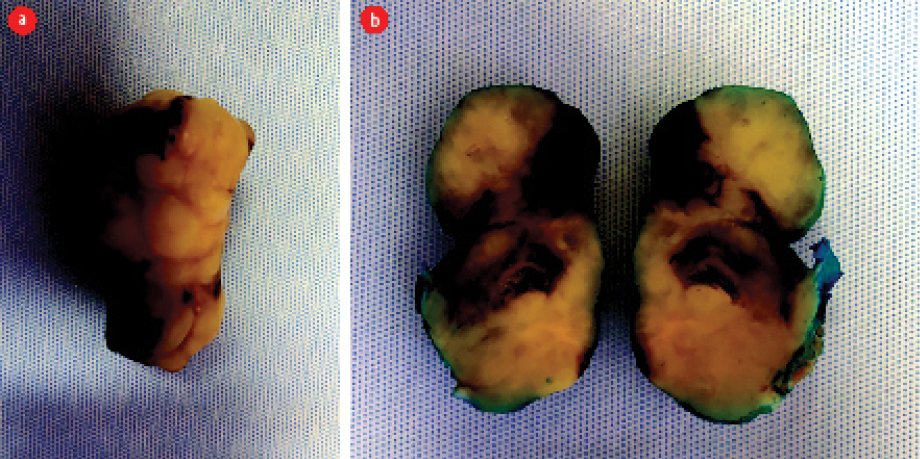 Figure 3: A resected, nodular, well-circumscribed, grey-white tumor. (a) Whole specimen. (b) Dissected specimen.
Figure 3: A resected, nodular, well-circumscribed, grey-white tumor. (a) Whole specimen. (b) Dissected specimen.
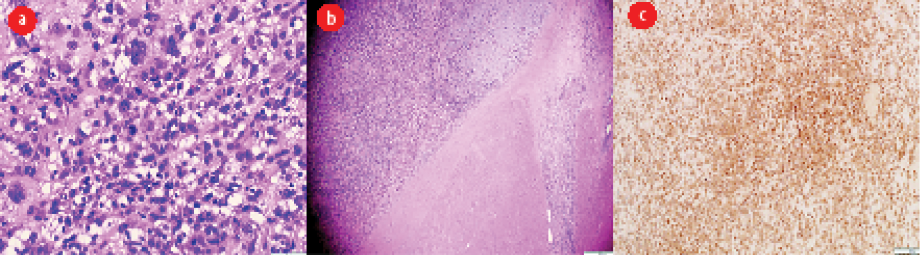 Figure 4: (a) Sheets of malignant cells with marked nuclear pleomorphism and scattered mitotic figures. (b) The malignant tumor invades the myocardium. (c) Immunohistochemistry profile shows positivity for mouse double minute 2.
Figure 4: (a) Sheets of malignant cells with marked nuclear pleomorphism and scattered mitotic figures. (b) The malignant tumor invades the myocardium. (c) Immunohistochemistry profile shows positivity for mouse double minute 2.
After the diagnosis of intimal sarcoma, the patient had a positron emission tomography-CT that revealed no evidence of distal metastasis. The patients received external beam radiotherapy using volumetric modulated arc therapy. A follow-up CT of the neck, chest, abdomen, and pelvis six months after treatment showed no evidence of local recurrence or distant metastasis. The patient was referred to the medical oncology clinic for further evaluation and he underwent four cycles of chemotherapy with cyclophosphamide, doxorubicin, and vincristine in combination.
Discussion
Cardiac intimal sarcomas are extremely rare tumors with few reported cases in the literature.5 The preoperative diagnosis of cardiac intimal sarcomas is very challenging, and they are usually misdiagnosed with thrombus or myxomas. Patients with cardiac intimal sarcomas can be asymptomatic or present with variable non-specific symptoms, depending on the location of the tumor, including valvular disease, heart failure, transit ischemic attack, and chest pain.5–8 In our case, the tumor was in the RVOT and protruding into the main PA during systole. This explains the clinical presentation of our patient with dizziness that was exacerbated by exercise and relieved by rest.
Histologically, intimal sarcoma is composed of atypical spindle cells that have variable degrees of atypia, mitotic activity, necrosis, and nuclear pleomorphism.3 Intimal sarcomas can rarely show areas with morphological features similar to angiosarcoma, rhabdomyosarcoma, and osteosarcoma.5 Immunohistochemically, tumor cells are usually positive for MDM2, vimentin, and osteopontin. Variable positivity is seen for BCL-2, CD117, P53, CD68, spinal muscular atrophy, and desmin. Although tumor cells are typically negative for factor VIII, CD34, and CD31, areas with angiosarcomatous differentiation can be positive.5
Different imaging modalities are used for the assessment of cardiac tumors. Echocardiogram is a readily available non-invasive diagnostic tool that is usually used as a first-line imaging tool for the assessment of cardiac tumors. It allows anatomical localization of cardiac tumors and the assessment of their relation to cardiac structures. However, echocardiogram is operator dependent with a restricted field of the view and limited ability to assess the right side of the heart. Moreover, echocardiogram is less sensitive for tissue characterization, and sometimes the differentiation between cardiac tumors and thrombi can be challenging.9 MRI is a well-established imaging tool for cardiac masses.4 It allows the differentiation of tumors from thrombi and further characterizing tumors based on their signals in different sequences. The inversion recovery technique acquired with a prolonged inversion time (> 600 ms) after contrast administration can accurately differentiate tumors from thrombus. Tumors usually have an intermediate signal intensity to the myocardium whereas thrombus appears dark. On late gadolinium enhancement sequence, thrombus does not enhance as it is avascular structure. On the other hand, the majority of cardiac tumors are usually hyperintense owing to their vascularity.4,10
Intimal sarcomas are highly aggressive, rapidly growing mesenchymal tumors with a very poor prognosis. The mean survival rate of patients with intimal sarcoma is three to 12 months.5,8 Surgical resection with clear margins is the mainstay in the management of cardiac sarcomas.7 Patients with complete resection live twice as long as patients without resection. However, complete resection is not always achievable as tumors might infiltrate vital structures.7,8 Postoperative chemotherapy has been reported to be effective in some cases but its role in the treatment of PA intimal sarcoma is not clearly defined. The same is true for radiation therapy and postoperative anticoagulation therapy. Our patient underwent resection of the tumor followed by radiation therapy and three cycles of chemotherapy including cyclophosphamide, doxorubicin, and vincristine in combination. Follow-up after six months revealed no evidence of recurrence of distance metastasis.
Conclusion
Intimal sarcomas are rare, highly aggressive, and rapidly growing mesenchymal tumors that can mimic benign cardiac tumors. Awareness of this rare entity and a high index of suspicion can help in early diagnosis and prompt surgical intervention.
Disclosure
The authors declared no conflicts of interest. Written consent was obtained from the patient.
references
- 1. Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J 2011;38(3):261-262.
- 2. Paraskevaidis IA, Michalakeas CA, Papadopoulos CH, Anastasiou-Nana M. Cardiac tumors. ISRN Oncol 2011;2011:208929.
- 3. Neuville A, Collin F, Bruneval P, Parrens M, Thivolet F, Gomez-Brouchet A, et al. Intimal sarcoma is the most frequent primary cardiac sarcoma: clinicopathologic and molecular retrospective analysis of 100 primary cardiac sarcomas. Am J Surg Pathol 2014 Apr;38(4):461-469.
- 4. Motwani M, Kidambi A, Herzog BA, Uddin A, Greenwood JP, Plein S. MR imaging of cardiac tumors and masses: a review of methods and clinical applications. Radiology 2013 Jul;268(1):26-43.
- 5. Ibrahim A, Luk A, Singhal P, Wan B, Zavodni A, Cusimano RJ, et al. Primary intimal (spindle cell) sarcoma of the heart: a case report and review of the literature. Case Rep Med 2013;2013:461815.
- 6. Valecha G, Pau D, Nalluri N, Liu Y, Mohammad F, Atallah JP. Primary intimal sarcoma of the left atrium: an incidental finding on routine echocardiography. Rare Tumors 2016 Nov;8(4):6389.
- 7. Li Z, Hsieh T, Salehi A. Recurrent cardiac intimal (spindle cell) sarcoma of the left atrium. J Cardiothorac Vasc Anesth 2013 Feb;27(1):103-107.
- 8. Grant L, Morgan I, Sumathi V, Salmons N. Intimal sarcoma of the left atrium presenting with transient ischaemic attack - a case report and review of the literature. J Cardiol Cases 2019 Nov;21(3):89-92.
- 9. Malik SB, Chen N, Parker RA III, Hsu JY. Transthoracic echocardiography: pitfalls and limitations as delineated at cardiac CT and MR imaging. Radiographics 2017 Mar-Apr;37(2):383-406.
- 10. The European Association of Cardiovascular Imaging (EACVI). EACVI CMR pocket guides. [cited 2020 August 7]. Available from: http://www.cmr-guide.com/Page102.html.