Outpatient parenteral antimicrobial therapy (OPAT) was first described in 1974 in the US and since then has grown in popularity in many countries.1,2 Compared to traditional inpatient care, OPAT brings benefits such as better-quality care and shortened hospital stays resulting in greater savings, shorter waiting lists, greater availability of hospital beds, and greater patient comfort in maintaining daily activities, resulting in patient satisfaction.3
OPAT involves administering intravenous (IV) antimicrobials to patients with infectious diseases in outpatient care (hospital OPAT) or at home, by a nurse (homecare OPAT), or by themselves/relatives.4 These services are usually used in indications such as cystic fibrosis, infectious endocarditis, complicated urinary tract infections, bone and joint infections (BJIs), and skin and soft tissue infections (SSTIs).5 Early OPAT services focused on the timely dismissal of hospitalized infection patients who were stable in care, requiring only extensive parenteral antimicrobial therapy. Over the past two decades, the services have made concerted efforts to avoid hospitalization of many acutely infected patients.6–10
Following the original consensus statement issued in 1998, the recommendations for OPAT in the UK were regularly updated and the most recent recommendations were published in 2019 to keep pace with the changing scenario.11,12 Despite the late start and slower initial introduction, OPAT services have expanded significantly in the UK.13 Recently, the link between OPAT and antimicrobial stewardship (AMS) has been recognized. Thus, OPAT is diligently disseminated as part of the UK government’s AMS programme.14
However, the expansion of these services has resulted in significant differences in OPAT practices, supply models, and governance rules. In addition, compliance with the national OPAT practice recommendations is weak.13 Therefore, we conducted this study to review the model and duration of OPAT services provided by our hospital, and to understand the demographic and clinical profiles of the patients. We also assessed whether our OPAT services were in line with the recommendations for current good practice in the treatment of infections and have made recommendations to minimize shortcomings.
Methods
This retrospective study was performed in a 550-bed tertiary hospital in the UK providing care in all major specialties. The electronic healthcare records of all the patients who received OPAT services between January 2019 and March 2021 were analyzed. The stable patients with infectious diseases, belonging to either-sex, aged ≥ 18 years, and receiving outpatient IV antimicrobials were included in the study. Those receiving antimicrobials through oral and other parenteral routes than IV were excluded.
Inhouse OPAT services at our hospital began on 1 October 2020, before which these services used to be outsourced. The organizational aspects of our multidisciplinary OPAT team were based on the UK OPAT good practice recommendations and their subsequent updates.11,12 The team, comprising a clinical microbiologist, a physician, clinical pharmacists, and specialist nurses, catered to patients with infectious diseases who were referred by physicians from the inpatient hospital wards and outpatient clinics. A weekly multidisciplinary team meeting was held for review of symptoms, treatment, as well as inspection and care of patient equipment such as the catheters. Follow-up laboratory and radiological investigations were performed, if required.
The patients received antimicrobials through peripheral catheters or peripherally inserted central catheters (PICC lines), the latter being inserted by specialist radiologists. The choice of the catheter was dependent on the duration of therapy (short vs. long term) and the type of therapy (intermittent vs. continuous administration). The PICC lines were used if the therapy was required for more than seven days and continuous administration was advised by the treating physician.
We used one of the two models of antimicrobial administration: clinic and homecare OPAT, where the nurses administered the therapy at the infusion site and home, respectively. The former model was mainly used for mobile patients requiring short-term antimicrobials (less than four days), while the latter model was used for those with limited mobility.
Data related to demographics (age and sex), diagnosis, presence of comorbidities such as diabetes mellitus (DM), rheumatoid arthritis and immunosuppression, the status of microbiological examination (prior to and during OPAT), antimicrobials used, duration of therapy (planned and actually administered), antimicrobials changed after microbiological examination, radiological examinations including computed tomography and magnetic resonance imaging, and outcomes of infection were recorded. Adverse events (AEs) related to antibiotics requiring discontinuation of therapy, vascular access complications, and death were also noted.
The data was analyzed with IBM SPSS Statistics (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.). The normality of the continuous variables was tested with Shapiro-Wilk’s test and non-normality distributed data was represented as median interquartile range (IQR). The categorical variables were represented as frequencies (percentages). For the purpose of analysis, the patients were distributed into two groups: younger adults (< 65 years) and older adults (≥ 65 years). Comparison between continuous and categorical variables was done with Mann-Whitney U and chi-square test, respectively. A two-tailed p < 0.050 was considered statistically significant.
Table 1: Characteristics of patients receiving OPAT (N = 199).
|
Age, year (median (IQR))
|
74 (62–84)
|
-
|
|
Male
|
121
|
60.8
|
|
Comorbidities
|
|
|
|
Diabetes mellitus
|
70
|
35.2
|
|
Rheumatoid arthritis
|
9
|
4.5
|
|
Immunocompromised host
|
62
|
31.2
|
|
Catheter type
|
|
|
|
Peripheral catheter
|
96
|
48.2
|
|
PICC lines
|
103
|
51.8
|
|
Referred from
|
|
|
|
Inpatient wards
|
126
|
63.3
|
|
Outpatient clinics
|
73
|
36.7
|
|
Model of antimicrobial administration
|
|
Hospital OPAT
|
101
|
50.8
|
|
Home OPAT
|
98
|
49.2
|
|
Indications for OPAT
|
|
|
|
Bones and joints infection
|
77
|
38.7
|
|
Pulmonary infection
|
24
|
12.1
|
|
Infected prosthesis
|
24
|
12.1
|
|
Skin and soft tissue infection
|
20
|
10.1
|
|
Sepsis
|
19
|
9.5
|
|
Renal and urinary infections
|
8
|
4.0
|
|
Others
|
27
|
13.6
|
|
Investigations
|
|
|
|
Radiological
|
101
|
50.8
|
|
Microbiological
|
188
|
94.5
|
|
Timing of microbiological examination, n = 188
|
|
Prior to initiation of antimicrobials
|
142
|
75.5
|
|
Within two weeks of antimicrobials initiation
|
35
|
18.6
|
|
Two weeks after antimicrobials initiation
|
8
|
4.3
|
OPAT: outpatient parenteral antimicrobial therapy; IQR: Interquartile range; PICC: peripherally inserted central catheters.
Results
Over 27 months, a total of 199 patients received OPAT services. They were predominantly male (60.8%) with a median age of 74 (IQR = 62–84) years. The majority had been referred from inpatient wards (126; 63.3%). Half of the patients received hospital OPAT (101; 50.8%) through PICC lines (103; 51.8%). Among various indications requiring IV antimicrobial therapy, the most commonly observed were BJI (77; 38.7%), pulmonary infection (24; 12.1%), infected prosthesis (24; 12.1%), and SSTI (20; 10.1%). Microbiological and radiological examinations were performed in 188 (94.5%) and 101 (50.8%) patients, respectively. DM (70; 35.2%) was the most common comorbidity. Among those in which microbiological examination was performed, 142 (75.5%) patients had it prior to initiation of antimicrobials. While 35 (18.6%) and eight (4.3%) patients underwent microbiological examination within and after two weeks of the initiation of antimicrobials, respectively. However, only three (1.6%) patients underwent microbiological examination both prior to and after the initiation of antimicrobials [Table 1].
Among patients who underwent microbiological examination, 58 (30.9%) had no growth of microorganisms. Among those with the growth of microorganisms, Staphylococcus aureus (38; 20.2%) was most commonly isolated. The majority of the patients received a single antimicrobial (147; 73.9%). Flucloxacillin (49; 24.6%), teicoplanin (32; 16.1%), and piperacillin-tazobactam (19; 9.5%) were the most common single antimicrobial agents used. Of all the antimicrobials, any flucloxacillin combination (67; 33.7%) followed by any teicoplanin combination (49; 24.6%) were the most common. Though 188 (94.5%) patients underwent microbiological examination, only in 31 (16.5%) of those cases were antimicrobials changed after receiving the culture reports. The planned durations for the administration of the antimicrobials ranged 3–90 days, while the actual durations ranged 3–187 days. Two-thirds of patients (135; 67.8%) received OPAT for longer periods than planned. Five (2.5%) patients received antimicrobials for 100 days or more, and one for 187 days. A minority (34; 17.1%) received antimicrobials as per the planned durations, while 30 (15.1%) received OPAT for shorter periods than planned. Re-treatment was required for 30 (15.1%) patients. Twenty-eight (14.1%) patients developed AEs, of which those involving the gastrointestinal system 13 (46.4%) pre dominated [Table 2].
Table 2: Microbiological findings, antimicrobials used, and complications of OPAT (N = 199).
|
Microorganisms identified, n = 188
|
|
|
|
No growth
|
58
|
30.9
|
|
Staphylococcus aureus
|
38
|
20.2
|
|
Escherichia coli
|
18
|
9.6
|
|
Staphylococcal spp.
|
6
|
3.2
|
|
Streptococcal spp.
|
16
|
8.5
|
|
Pseudomonas spp.
|
12
|
6.4
|
|
Others
|
40
|
21.3
|
|
Antimicrobials used
|
|
|
|
Any flucloxacillin combination
|
67
|
33.7
|
|
Any teicoplanin combination
|
49
|
24.6
|
|
Any amoxicillin combination
|
18
|
9.0
|
|
Any ertapenem combination
|
10
|
5.0
|
|
Any ceftriaxone combination
|
9
|
4.5
|
|
Piperacillin/tazobactam
|
19
|
9.5
|
|
Others
|
27
|
13.6
|
|
Antimicrobials changed after microbiological examination, n = 188
|
31
|
16.5
|
|
Duration of antimicrobials prescribed
|
|
|
|
Planned, median (IQR)
|
42 (14–42)
|
-
|
|
Actual, median (IQR)
|
37 (15–51)
|
-
|
|
Duration of OPAT
|
|
|
|
Longer than planned
|
135
|
67.8
|
|
As planned
|
34
|
17.1
|
|
Shorter than planned
|
30
|
15.1
|
|
Complications
|
28
|
14.1
|
|
Gastrointestinal
|
13
|
46.4
|
|
Hematological
|
4
|
14.3
|
|
Others
|
11
|
39.3
|
|
Mortality after completion of OPAT
|
|
|
|
At 30 days
|
2
|
1.0
|
OPAT: outpatient parenteral antimicrobial therapy; IQR: interquartile range.
Of the 28 cases of AE, 25 were antimicrobials-related, while the remaining three were catheter-associated. Among the patients with AEs, six had diarrhea, three had vomiting, two each had nausea and lethargy, and one each had acute kidney injury, anemia, neutropenia, hyperkalemia, chest pain, raised alkaline phosphatase levels, insomnia, relapse of infection, septic infection, sepsis secondary to chest drains, septic emboli, and death. Catheter-associated AEs were related to line blockage, swelling of skin adjacent to the catheter, and thrombophlebitis in one patient each. Two patients died after 30 days of completing OPAT, and after one year, this number rose to 18. The deaths were related to relapse of pulmonary infection in eight patients, malignancy in five, and sepsis in five patients [Table 2].
Between the younger and older groups, there were no significant differences in characteristics such as sex, comorbidities, catheter type, referral status, model of antimicrobial administration, indications for OPAT, or types of investigations conducted (p > 0.050) [Table 3].
Table 3: Comparison of characteristics of patients receiving OPAT.
|
Age, year (median (IQR))
|
55 (50–60)
|
80 (72–85)
|
< 0.001
|
|
Male
|
33 (61.1)
|
88 (60.7)
|
0.957*
|
|
Comorbidities
|
|
|
|
|
Diabetes mellitus
|
22 (40.7)
|
48 (33.1)
|
0.316*
|
|
Rheumatoid arthritis
|
4 (7.4)
|
5 (3.4)
|
0.232*
|
|
Immunocompromised host
|
12 (22.2)
|
50 (34.5)
|
0.097*
|
|
Catheter type
|
|
|
|
|
Peripheral catheter
|
26 (48.1)
|
70 (48.3)
|
0.987*
|
|
PICC lines
|
28 (51.9)
|
75 (51.7)
|
|
|
Referred from
|
|
|
|
|
Inpatient wards
|
36 (66.7)
|
90 (62.1)
|
0.550*
|
|
Outpatient clinics
|
18 (33.3)
|
55 (37.9)
|
|
|
Model of antimicrobial administration
|
|
|
|
|
Hospital OPAT
|
29 (53.7)
|
72 (49.7)
|
0.611*
|
|
Home OPAT
|
25 (46.3)
|
73 (50.3)
|
|
|
Indications for OPAT
|
|
|
|
|
Bones and joints infection
|
21 (38.9)
|
56 (38.6)
|
0.798*
|
|
Pulmonary infection
|
5 (9.3)
|
19 (13.1)
|
0.259*
|
|
Skin and soft tissue infection
|
4 (7.4)
|
16 (11.0)
|
|
|
Infected prosthesis
|
7 (13.0)
|
17 (11.7)
|
0.811*
|
|
Sepsis
|
4 (7.4)
|
15 (10.3)
|
0.879*
|
|
Renal and urinary infections
|
3 (5.6)
|
5 (3.4)
|
|
|
Others
|
10 (18.5)
|
17 (11.7)
|
0.213*
|
|
Investigations
|
|
|
|
|
Radiological
|
31 (57.4)
|
70 (48.3)
|
0.252*
|
|
Microbiological
|
51 (94.4)
|
137 (94.5)
|
0.992*
|
|
Timing of microbiological examination
|
|
|
|
|
Prior to initiation of OPAT
|
41 (80.4)
|
101 (73.7)
|
0.140*
|
|
Within two weeks of OPAT initiation
|
9 (17.6)
|
26 (19.0)
|
0.344*
|
|
Two weeks after antimicrobials initiation
|
1 (2.0)
|
7 (5.1)
|
|
OPAT: outpatient parenteral antimicrobial therapy; IQR: interquartile range; PICC: peripherally inserted central catheters.
*Chi-square test.
Significantly more older adults had an infection due to S. aureus (p < 0.001), and Escherichia coli, Staphylococcal spp., Streptococcal spp., and Pseudomonas spp. (p = 0.003). However, there was no significant difference between younger and older adults in terms of other isolated microorganisms, antimicrobials used, duration of antimicrobials prescribed, and OPAT AEs (p > 0.050). Though more older adults had mortality both after 30 days and one year, this did not reach a statistically significant level (p = 0.069) [Table 4].
Table 4: Comparison of microbiological findings, antimicrobials used, and complications of OPAT.
|
Microorganisms identified
|
|
|
|
|
No growth
|
14 (27.5)
|
44 (32.1)
|
0.538*
|
|
Staphylococcus aureus
|
20 (39.2)
|
18 (13.1)
|
< 0.001*
|
|
Escherichia coli
|
1 (2.0)
|
17 (12.4)
|
0.003*
|
|
Staphylococcal spp.
|
1 (2.0)
|
5 (3.6)
|
-
|
|
Streptococcal spp.
|
3 (5.9)
|
13 (9.5)
|
-
|
|
Pseudomonas spp.
|
1 (2.0)
|
11 (8.0)
|
-
|
|
Others
|
11 (21.6)
|
29 (21.2)
|
0.952*
|
|
Antimicrobials used
|
|
|
|
|
Any flucloxacillin combination
|
23 (42.6)
|
44 (30.3)
|
0.104*
|
|
Any teicoplanin combination
|
10 (18.5)
|
39 (26.9)
|
0.223*
|
|
Any amoxicillin combination
|
4 (7.4)
|
14 (9.7)
|
0.987*
|
|
Any ertapenem combination
|
3 (5.6)
|
7 (4.8)
|
-
|
|
Any ceftriaxone combination
|
3 (5.6)
|
6 (4.1)
|
-
|
|
Piperacillin/tazobactam
|
5 (9.3)
|
14 (9.7)
|
0.933*
|
|
Others
|
6 (11.1)
|
21 (14.5)
|
0.537*
|
|
Antimicrobials changed after microbiological examination
|
7 (12.9)
|
24 (16.6)
|
0.535*
|
|
Duration of antimicrobials prescribed
|
|
|
|
|
Planned, median (IQR)
|
42 (14–42)
|
42 (14–42)
|
0.771$
|
|
Actual, median (IQR)
|
41 (15–51)
|
36 (14–51)
|
0.569$
|
|
Antimicrobials for duration more than planned
|
29 (53.7)
|
77 (53.1)
|
0.940*
|
|
Complications
|
|
|
|
|
Gastrointestinal
|
5 (9.3)
|
8 (5.5)
|
0.342*
|
|
Hematological
|
1 (1.9)
|
3 (2.1)
|
0.575*
|
|
Others
|
4 (7.4)
|
7 (4.8)
|
-
|
|
Mortality after completion of OPAT
|
|
|
|
|
At 30 days
|
0 (0.0)
|
2 (1.4)
|
0.069*
|
OPAT: outpatient parenteral antimicrobial therapy; IQR: interquartile range.
*Chi-square test; $Mann-Whitney U.
The median actual OPAT duration was significantly greater than the median planned OPAT duration for the total study population (p < 0.001), younger adults (p = 0.031) and older adults (p = 0.002) [Figure 1].
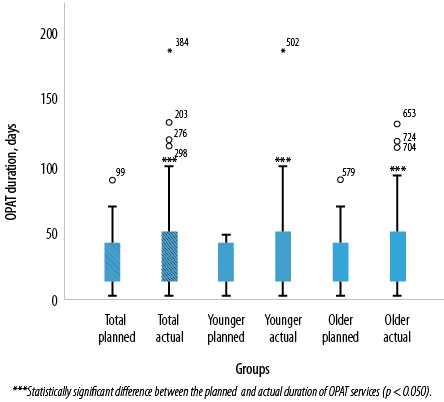 Figure 1: Association between planned and actual duration of outpatient parenteral antimicrobial therapy (OPAT) in the total study population, younger adults, and older adults.
Figure 1: Association between planned and actual duration of outpatient parenteral antimicrobial therapy (OPAT) in the total study population, younger adults, and older adults.
No statistically significant association was found between cases of death and variables of sex, comorbidities (DM, rheumatoid arthritis, and immunosuppression), microbiological examination prior to antimicrobials, continuation of antimicrobial therapy longer than planned, AEs, PICC lines, referral from inpatient wards, or using hospital OPAT service (all p > 0.050) [Table 5].
Table 5: Association between death and characteristics of patients who died one year after cessation of OPAT.
|
Male
|
11 (61.1)
|
110 (60.8)
|
121
|
0.978
|
|
Diabetes mellitus
|
10 (55.6)
|
60 (33.1)
|
70
|
0.058
|
|
Rheumatoid arthritis
|
1 (5.6)
|
8 (4.4)
|
9
|
0.825
|
|
Immunosuppression
|
9 (50.0)
|
53 (29.3)
|
62
|
0.070
|
|
Microbiological examination prior to OPAT
|
12 (66.7)
|
130 (71.8)
|
142
|
0.266
|
|
Antimicrobials further than planned
|
8 (44.4)
|
98 (54.1)
|
106
|
0.432
|
|
Adverse events
|
0 (0.0)
|
28 (15.5)
|
28
|
NA
|
|
PICC lines
|
7 (38.9)
|
96 (53.0)
|
103
|
0.252
|
|
Referral from inpatient wards
|
9 (50.0)
|
117 (64.6)
|
126
|
0.219
|
OPAT: outpatient parenteral antimicrobial therapy; PICC: peripherally inserted central catheters.
*Chi-square test.
Discussion
While antimicrobial agents are indispensable in treating severe and potentially fatal infections,15 their injudicious use can lead to negative outcomes. Some antimicrobials (aminoglycosides) used in combinations with other antimicrobials (amphotericin) or other classes of drugs produce toxic AEs. Indiscriminate use of broad-spectrum antimicrobials disrupts the normal body flora, thereby permitting proliferation of resistant and opportunistic microorganisms which can lead to secondary infections.16 Thus, ideally, the pathogen should be identified and its virulence estimated before initiating the antimicrobial therapy and planning its duration.
The OPAT has become common in Canada and the UK. It is also practiced in various forms in some countries in South America, Europe, and the Asia Pacific.1,17–20 Several health care centers across Australia and New Zealand also provide OPAT services.21–24 In Asia, the rising popularity of OPAT is marred by lax regulations. One comprehensive survey found that 97/171 (57%) healthcare facilities across 17 Asian countries were not following the recommended OPAT practices.25
Our hospital in Surrey, England, follows the UK national recommendations which require OPAT services to be run by a multidisciplinary team with at least one physician, an infectious disease specialist, a specialist nurse, and a clinical antimicrobial pharmacist.12 In this study, we have analyzed our OPAT services provided between January 2019 and March 2021.
Over the course of 27 months, 199 patients were treated with OPAT. This saved 7514 bed-days, benefiting both patients and healthcare. The majority of these patients were referred from inpatient wards which reflects the actual needs of inpatient centers for beds. Our results are consistent with those reported in other studies.26,27 Therefore, extended OPAT services lead to shorter hospital stays, particularly beneficial for hospitals with high bed occupancy. In addition, these services improve patient satisfaction.28,29
BJI together with infected prosthesis were the dominant indications that required antimicrobial therapy. Other studies also have reported a high prevalence of these infections.27,30 BJI and SSTI lead to significant numbers of hospital admissions with longer hospital stays.31 Prosthetic joint infections, although infrequent, carry 2–3 times higher risk of revision surgery. With the growing population of older people, the proportion of joint prostheses is expected to increase exponentially, along with cases of prosthetic joint infection.32 Thus, the number of elderly patients requiring OPAT services is expected to increase.
Our OPAT team treated patients who were predominantly infected with S. aureus and E. coli. Among the other isolated microorganisms, one patient each had infection with Clostridium difficile and methicillin-resistant S. aureus. The infection-prone factors in the patient population were advanced age, most patients being aged ≥ 65 years, and mostly affected by immunosuppression due to comorbidities such as DM, steroid use, and cancers. Antimicrobial resistance is a growing international health problem,33,34 further increasing the demand for parenteral antimicrobials. OPAT services need to develop more flexibility and readiness to address difficult-to-treat infections and new scenarios arising from resistant gram-negative bacterial infections.
We observed that flucloxacillin, teicoplanin, and their combinations with other antimicrobials were most often used. Similar results have been reported by other studies.35,36 Although hospital OPAT was the dominant model, we observed greater acceptance of home OPAT model in the last year. However, none of the patients felt comfortable with the self-treating OPAT model. This was basically due to the relatively new OPAT set-up in our hospital, and as a confidence-building measure, the OPAT group actively supported the home model instead of the self-rationing model, which requires training and supervision.
In case of infections with rapid clinical improvement, traditional long-acting IV antimicrobials may not always be necessary and an early transition from IV to oral treatment is possible. Long-term antimicrobial therapy is associated with a higher risk of resistance.37 AMS is essential to prevent the risk of routine continuance OPAT for far longer than originally envisaged.38,39 Indeed, we observed that many patients—both younger and older adults—were receiving OPAT significantly longer than planned. This is supported by reports elsewhere that patients treated partially or entirely at home may receive longer-term therapy than those treated entirely in hospital.38
Only three-quarters of our OPAT patients underwent microbiological tests before antimicrobials were administered. It is worth noting that only a fraction of patients had their antimicrobials changed after receiving the culture report. Although multidisciplinary team meetings were held weekly after the initiation of OPAT inpatient care to review patients’ symptoms and treatment, retrospective analysis and internal discussion records showed that only 29% of patients were treated as per standard OPAT recommendations on appropriate testing, antibiotic selection, and treatment duration. These were primarily attributed to temporary staffing and inadequate sharing of documentation among the OPAT multidisciplinary team. Another factor was that OPAT services were being outsourced before October 2020 with an attendant lack of monitoring of services. In addition, the contribution of the 2019 coronavirus outbreak to the functioning of OPAT team and attitudes of patients toward prolonged antimicrobial therapy could not be ignored, as it resulted in many patients missing clinic appointments or follow-ups, leading to prolonged antimicrobial therapy.
A few other studies have compared the OPAT characteristics and results in younger and older adults. We did not notice any significant differences between them except for the significantly greater median age and infections with S. aureus, E. coli, Staphylococcal spp., Streptococcal spp., and Pseudomonas spp. in older adults. Other studies reported similar results.30,40 Brzozowski et al,30 showed no difference between the younger and older patients in terms of AEs or access to health care within 30 days of OPAT cessation. Mujal et al,40 reported that the rates of antibiotic treatment and re-hospitalization in younger and older patients were the same due to poor control of underlying infection, however, older adults had a higher rate of rehospitalization resulting from the exacerbation of the underlying diseases. In addition, AEs and catheter-related complications were identical across the age groups.
No statistically significant association was found between mortality and the different characteristics studied. Salles et al,41 also reported lack of significant association between mortality and factors such as age group, sex, type of infection, OPAT model, type of catheter, or microbiological test used to guide treatment. Mortality, however, was significantly associated with palliative care and post-enrolment physician visit. Shrestha et al,42 who studied nonagenarians receiving OPAT services, found a significant association between mortality and age, as well as Clostridioides difficile infection, higher white blood cell count, and lower platelet count at hospital admission. These parameters were not part of our study, so we could not assess such association.
Our study has limitations. First, being a retrospective study dependent on past healthcare records, it was not possible to randomize patients by age group. Second, we could not find accurate records of sensitivity reports, source data for various microbiological samples, or decisions taken to extend OPAT. Third, no data was available on other comorbidities that might have influenced mortality or AEs. The lack of data on immunomodulatory drugs meant that no drug-drug interactions leading to failure of antimicrobial therapy could be identified. Fourth, the retrospective nature of the study meant that we were unable to assess patient satisfaction, while the lack of data on treatment costs prevented cost analysis. Fifth, being a single-center study, the results cannot be generalized. Finally, no microorganisms were isolated from 30.9% of patient samples. Thus, there is a high probability that infection was not the cause of these patients’ presentation.
The results of this study suggest that much more needs to be done to achieve the recommended level of OPAT functioning. We make the following recommendations: First, the antibiotic registry should be easily accessible and available in the form of a single digital registry that includes antimicrobials prescribed in both inpatient and outpatient settings. These records should include patient details including diagnosis, comorbidities, specialties treating the patient, name of prescriber and administrator, antimicrobials prescribed with their rationale, dose, route, frequency and AEs, microbiological tests and their results, planned duration of treatment, and actual duration of treatment (start and end dates). Second, active surveillance should be carried out at regular intervals. Monitoring should focus on the following: (1) microbiological, hematological, and radiological examinations; (2) notification of the examination results to the referring or responsible physician; and (3) the maintenance and follow-up of a digital record containing the physician’s comments on the results of the examinations and the estimated duration of any additional antimicrobial treatment. Third, an integrated outpatient system should be established in hospitals offering OPAT services. This system should alert all collaborating physicians when a patient arrives at the hospital, either in the emergency department or in the outpatient department, especially if the patient is receiving IV antimicrobials. Cooperation should be enhanced between physicians responsible for administering antimicrobials and physicians monitoring the patient for other conditions, the OPAT team, and general practitioners.
Conclusion
The need and relevance of OPAT services are increasing with the growing public acceptance of the home model. This study suggests that the actual duration of OPAT services was significantly longer than planned and that the principles of AMS were less adhered to. In terms of different parameters, there was no significant difference between younger and older adults, except for older adults having a higher incidence of S. aureus, E. coli, Staphylococcus spp., Streptococcus spp., and Pseudomonas spp. infections. OPAT was found to be safe for both younger and older adults, with no significant association between mortality and patient characteristics.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgments
The authors would like to thank Dr. Vikas S. Sharma, Principal Consultant, Maverick Medicorum® (India), for statistical analysis and medical writing assistance in the preparation of this article.
references
- 1. Rucker RW, Harrison GM. Outpatient intravenous medications in the management of cystic fibrosis. Pediatrics 1974 Sep;54(3):358-360.
- 2. Esposito S, Noviello S, Leone S, Tice A, Seibold G, Nathwani D, et al; International OPAT Registry. Outpatient parenteral antibiotic therapy (OPAT) in different countries: a comparison. Int J Antimicrob Agents 2004 Nov;24(5):473-478.
- 3. Ravelingien T, Buyle F, Deryckere S, Sermijn E, Debrauwere M, Verplancke K, et al. Optimization of a model of out-of-hospital antibiotic therapy (OPAT) in a Belgian university hospital resulting in a proposal for national implementation. Acta Clin Belg 2016 Oct;71(5):297-302.
- 4. Chapman AL. Outpatient parenteral antimicrobial therapy. BMJ 2013 Mar;346:f1585.
- 5. Steffens E, Quintens C, Derdelinckx I, Peetermans WE, Van Eldere J, Spriet I, et al. Outpatient parenteral antimicrobial therapy and antibiotic stewardship: opponents or teammates? Infection 2019 Apr;47(2):169-181.
- 6. Kieran J, O’Reilly A, Parker J, Clarke S, Bergin C. Self-administered outpatient parenteral antimicrobial therapy: a report of three years experience in the Irish healthcare setting. Eur J Clin Microbiol Infect Dis 2009 Nov;28(11):1369-1374.
- 7. Wai AO, Frighetto L, Marra CA, Chan E, Jewesson PJ. Cost analysis of an adult outpatient parenteral antibiotic therapy (OPAT) programme. A Canadian teaching hospital and Ministry of Health perspective. Pharmacoeconomics 2000 Nov;18(5):451-457.
- 8. Tice AD, Rehm SJ, Dalovisio JR, Bradley JS, Martinelli LP, Graham DR, et al; IDSA. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis 2004 Jun;38(12):1651-1672.
- 9. Laupland KB, Gill MJ, Schenk L, Goodwin D, Davies HD. Outpatient parenteral antibiotic therapy: evolution of the Calgary adult home parenteral therapy program. Clin Invest Med 2002 Oct;25(5):185-190.
- 10. Seaton RA, Sharp E, Bezlyak V, Weir CJ. Factors associated with outcome and duration of therapy in outpatient parenteral antibiotic therapy (OPAT) patients with skin and soft-tissue infections. Int J Antimicrob Agents 2011 Sep;38(3):243-248.
- 11. Chapman AL, Seaton RA, Cooper MA, Hedderwick S, Goodall V, Reed C, et al; BSAC/BIA OPAT Project Good Practice Recommendations Working Group. Good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults in the UK: a consensus statement. J Antimicrob Chemother 2012 May;67(5):1053-1062.
- 12. Chapman AL, Patel S, Horner C, Green H, Guleri A, Hedderwick S, et al. Updated good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults and children in the UK. JAC Antimicrob Resist 2019 Aug;1(2):dlz026.
- 13. Durojaiye OC, Cartwright K, Ntziora F. Outpatient parenteral antimicrobial therapy (OPAT) in the UK: a cross-sectional survey of acute hospital trusts and health boards. Diagn Microbiol Infect Dis 2019 Jan;93(1):58-62.
- 14. Ashiru-Oredope D, Sharland M, Charani E, McNulty C, Cooke J; ARHAI Antimicrobial Stewardship Group. Improving the quality of antibiotic prescribing in the NHS by developing a new antimicrobial stewardship programme: start smart–then focus. J Antimicrob Chemother 2012 Jul;67(Suppl 1):i51-i63.
- 15. Mohsen S, Dickinson JA, Somayaji R. Update on the adverse effects of antimicrobial therapies in community practice. Can Fam Physician 2020 Sep;66(9):651-659.
- 16. Weledji EP, Weledji EK, Assob JC, Nsagha DS. Pros, cons and future of antibiotics. New Horiz Transl Med 2017;4(1-4):9-14.
- 17. Upton A, Ellis-Pegler RB, Woodhouse A. Outpatient parenteral antimicrobial therapy (OPAT): a review of experience at Auckland Hospital. N Z Med J 2004 Aug;117(1200):U1020.
- 18. Grayson ML, Silvers J, Turnidge J. Home intravenous antibiotic therapy. A safe and effective alternative to inpatient care. Med J Aust 1995 Mar;162(5):249-253.
- 19. Fisher DA, Kurup A, Lye D, Tambyah PA, Sulaiman Z, Poon EY, et al. Outpatient parenteral antibiotic therapy in Singapore. Int J Antimicrob Agents 2006 Dec;28(6):545-550.
- 20. Nathwani D, Zambrowski JJ; AdHOC Workshop. Advisory group on home-based and outpatient care (AdHOC): an international consensus statement on non-inpatient parenteral therapy. Clin Microbiol Infect 2000 Sep;6(9):464-476.
- 21. Tran A, Taylor DM. Medical model for hospital in the home: effects on patient management. Aust Health Rev 2009 Aug;33(3):494-501.
- 22. Subedi S, Looke DF, McDougall DA, Sehu MM, Playford EG. Supervised self-administration of outpatient parenteral antibiotic therapy: a report from a large tertiary hospital in Australia. Int J Infect Dis 2015 Jan;30:161-165.
- 23. White HA, Davis JS, Kittler P, Currie BJ. Outpatient parenteral antimicrobial therapy-treated bone and joint infections in a tropical setting. Intern Med J 2011 Sep;41(9):668-673.
- 24. Ingram PR, Cerbe L, Hassell M, Wilson M, Dyer JR. Limited role for outpatient parenteral antibiotic therapy for community-acquired pneumonia. Respirology 2008 Nov;13(6):893-896.
- 25. Fisher D, Michaels J, Hase R, Zhang J, Kataria S, Sim B, et al. Outpatient parenteral antibiotic therapy (OPAT) in Asia: missing an opportunity. J Antimicrob Chemother 2017 Apr;72(4):1221-1226.
- 26. Allison GM, Muldoon EG, Kent DM, Paulus JK, Ruthazer R, Ren A, et al. Prediction model for 30-day hospital readmissions among patients discharged receiving outpatient parenteral antibiotic therapy. Clin Infect Dis 2014 Mar;58(6):812-819.
- 27. Hase R, Yokoyama Y, Suzuki H, Uno S, Mikawa T, Suzuki D, et al. Review of the first comprehensive outpatient parenteral antimicrobial therapy program in a tertiary care hospital in Japan. Int J Infect Dis 2020 Jun;95:210-215.
- 28. Durojaiye OC, Kritsotakis EI, Johnston P, Kenny T, Ntziora F, Cartwright K. Developing a risk prediction model for 30-day unplanned hospitalization in patients receiving outpatient parenteral antimicrobial therapy. Clin Microbiol Infect 2019 Jul;25(7):905.e1-905.e7.
- 29. Saillen L, Arensdorff L, Moulin E, Voumard R, Cochet C, Boillat-Blanco N, et al. Patient satisfaction in an outpatient parenteral antimicrobial therapy (OPAT) unit practising predominantly self-administration of antibiotics with elastomeric pumps. Eur J Clin Microbiol Infect Dis 2017 Aug;36(8):1387-1392.
- 30. Brzozowski K, Datta R, Canterino J, Malinis M, Juthani-Mehta M. Adverse events and healthcare utilization associated with outpatient parenteral antimicrobial therapy among older versus younger adults. Open Forum Infect Dis 2020 Aug;7(10):ofaa358.
- 31. Howell A, Parker S, Tsitskaris K, Oddy MJ. The burden of bone, native joint and soft tissue infections on orthopaedic emergency referrals in a city hospital. Ann R Coll Surg Engl 2016 Jan;98(1):34-39.
- 32. Ahmed SS, Haddad FS. Prosthetic joint infection. Bone Joint Res 2019 Dec;8(11):570-572.
- 33. Naylor NR, Pouwels KB, Hope R, Green N, Henderson KL, Knight GM, et al. The health and cost burden of antibiotic resistant and susceptible Escherichia coli bacteraemia in the English hospital setting: a national retrospective cohort study. PLoS One 2019 Sep;14(9):e0221944.
- 34. Hu XY, Logue M, Robinson N. Antimicrobial resistance is a global problem - a UK perspective. Eur J Integr Med 2020 Jun;36:101136.
- 35. Pensotti C, Nacinovich F, Vidiella G, Carbone E, Marin M, Di Stéfano C, et al. (Teicoplanin in the treatment of bone and joint infections due to methicillin resistant staphylococci. Experience in adult patients). Medicina (B Aires) 2002;62(Suppl 2):40-47.
- 36. Mackintosh CL, White HA, Seaton RA. Outpatient parenteral antibiotic therapy (OPAT) for bone and joint infections: experience from a UK teaching hospital-based service. J Antimicrob Chemother 2011 Feb;66(2):408-415.
- 37. McMullan BJ, Andresen D, Blyth CC, Avent ML, Bowen AC, Britton PN, et al; ANZPID-ASAP group. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis 2016 Aug;16(8):e139-e152.
- 38. Bryant PA, Katz NT. Inpatient versus outpatient parenteral antibiotic therapy at home for acute infections in children: a systematic review. Lancet Infect Dis 2018 Feb;18(2):e45-e54.
- 39. Mace AO, McLeod C, Yeoh DK, Vine J, Chen YP, Martin AC, et al. Dedicated paediatric outpatient parenteral antimicrobial therapy medical support: a pre-post observational study. Arch Dis Child 2018 Feb;103(2):165-169.
- 40. Mujal A, Sola J, Hernandez M, Villarino MA, Baylina M, Tajan J, et al. Safety and effectiveness of outpatient parenteral antimicrobial therapy in older people. J Antimicrob Chemother 2016 May;71(5):1402-1407.
- 41. Salles TC, Cerrato SG, Santana TF, Medeiros EA. Factors associated with successful completion of outpatient parenteral antibiotic therapy in an area with a high prevalence of multidrug-resistant bacteria: 30-day hospital admission and mortality rates. PLoS One 2020 Nov;15(11):e0241595.
- 42. Shrestha NK, Blaskewicz C, Gordon SM, Everett A, Rehm SJ. Safety of outpatient parenteral antimicrobial therapy in Nonagenarians. Open Forum Infect Dis 2020 Oct;7(10):ofaa398.