The term cancer encompasses a wide range of disorders that can affect any organ in the body.1 Cancer occurs when some cells in the body proliferate uncontrollably and sometimes spread to other parts of the body, invading organs.2 It is a major cause of death worldwide and a significant impediment to extending human life expectancy. In 2020, an estimated 19.3 million new cancer cases were diagnosed with ten million deaths worldwide. India reported around 1.3 million new cases and 0.85 million fatalities.3 More than 100 types of cancers have been identified. Cancer types are generally named after the organs or tissues where tumors originate. Cancers can also be categorized based on the cell type that caused them, such as squamous or epithelial cells.4
Lung cancer is one of the most prevalent cancers in the world, both in terms of mortality and incidence, with approximately one million new cases diagnosed every year.5 It is the foremost cause of cancer death in both sexes, accounting for over a quarter (27%) of all cancer-related deaths.6 In 2020, a projected 2.2 million new cases of lung cancer were reported worldwide, representing 11.4% of the global cancer burden. Lung cancer claimed over 1.8 million lives in 2020.1
Non-small cell lung cancer (NSCLC) accounts for 75–85% of all lung cancer diagnoses, which includes adenocarcinoma, squamous cell carcinoma, and large-cell carcinoma. Several factors such as the stage, grade, and functional capacity of the patient influence the treatment options. Surgery, chemotherapy, and chemoradiotherapy are usually used to treat early- and middle-stage NSCLC. According to reports, stage I NSCLC patients have a 5-year survival rate of 77%, which declines to 23% at stage IIIa. Surgery, chemotherapy, immunotherapy, and radiotherapy are used to enhance life expectancy at advanced stages of NSCLC.7
It is estimated that nearly 70% of patients with advanced NSCLC attain disease stabilization or clinical remission with the first-line platinum-based treatment. The rest advance to the point where they require second-line treatment. Monotherapy using docetaxel, erlotinib, or pemetrexed is the currently authorized second-line therapy for NSCLC.8 Docetaxel is a plant alkaloid that is well tolerated by individuals with metastatic or advanced NSCLC and is approved in several countries as the first-line therapy in combination with cisplatin as a second-line monotherapy or as single-agent maintenance therapy. Also, for such patients, docetaxel in combination with a platinum-based agent (carboplatin or cisplatin) is typically regarded as the first-line therapy.9
The number of months between the completion of the randomization trial and mortality for any reason is referred to as the overall survival (OS).10 Randomized trials are regarded as the most suitable and accurate method of determining the efficacy and safety of new clinical methods and drugs. By subjecting docetaxel to randomized control trials (RCTs), researchers learned more about the effects on patients of this drug in various combinations, such as with antineoplastic drugs, kinase inhibitors, and monoclonal antibodies.11 Although docetaxel has been shown to be a good choice for second-line therapy, its OS benefit is modest. When antineoplastic drugs, kinase inhibitors, and monoclonal antibodies were compared to docetaxel as a second-line therapy, no survival benefit was seen.12
Methods
The major goal of this analysis was to ascertain the OS of advanced NSCLC of docetaxel-based second-line treatment by evaluating RCTs in the literature. We used Google Scholar, Science Direct, Scopus, PubMed, and the Cochrane library databases to conduct an extensive literature search for articles published between 2010 and 2021. ‘Advanced NSCLC’, ‘randomized control trial’, ‘chemotherapy’, ‘docetaxel’, and ‘second-line treatment’ were among the terms included in our search.
The study had the following inclusion criteria: (a) randomized trials comparing docetaxel to kinase inhibitors, antineoplastic agents, and monoclonal antibodies as second-line chemotherapy for patients with clinically proven advanced NSCLC; (b) the standard treatment given should be a docetaxel-based second-line treatment; (c) the study should be a phase II or phase III trial; and (d) the chief outcome measure should be OS of the patient. Exclusion criteria were: (a) studies that compared docetaxel to drug classes other than kinase inhibitors, antineoplastic agents, and monoclonal antibodies; (b) earlier studies from the same author with overlapping data (to eliminate publication bias); and (c) editorials, case reports, conference articles, experimental studies, and other related studies that failed to deliver comprehensive results. Based on these criteria, the applicable clinical trials were manually chosen.
The first author, year of publication, trial information, demographic parameters, histological characteristics, smoking status, treatment for each group, and adverse events were all extracted using a fixed standardized procedure. In this study, docetaxel was considered the standard treatment arm. Kinase inhibitor, antineoplastic agent, and monoclonal antibody were taken as the intervention arm.
The methodological quality of the trials was classified according to the modified Jadad score. Several steps were taken to reduce publication bias. These included adopting a comprehensive search strategy, selecting publications strictly based on inclusion criteria, and strict application of exclusion criteria to filter out unsuitable ones. Manual strategies were also used to detect and avoid publication bias. A funnel plot tested the impact of these strategies.
The effect size was calculated using the standardized mean difference and then corrected using Hedge’s approach. Hedge’s g value was then used to symbolize it. The Random-effect model was used to pool the effect sizes. Higgins and Thompson’s I2 statistics were used for heterogenicity estimation. A Forest plot enabled us to graphically summarize the meta-analysis results, using R Studio’s Meta package version 4.1.1.
Results
The flow chart for study inclusion and exclusion criteria in the meta-analysis of drug intervention prevalence is shown in Figure 1. The database search yielded 1009 publications retrieved from Google Scholar, PubMed, Science Direct, Scopus, and Cochrane databases. After removing duplicate results, 984 papers remained, and 435 were retained after removing articles related to non-RCTs. Because of phase I RCTs, 36 were ruled out, while 53 were ruled out because these were not primarily dealing with NSCLC. Following the screening process, 278 articles were left for full-text examination. Again, 156 articles were removed due to the absence of docetaxel in the treatment arm. Another 101 publications were excluded due to the absence of monoclonal antibodies, kinase inhibitors, or antineoplastic drugs in the intervention arm. Finally, a total of 18 RCTs were chosen for this study.
Table 1 shows the baseline characteristics of 18 RCTs, including study name, first author, publication year, phase trial, number of recruited patients, intervention and control groups, and outcome data. This meta-analysis includes a total of 9738 patients distributed among 18 RCTs. Six RCTs involved 2160 patients in an antineoplastic agent (intervention arm), seven with 4090 patients in the kinase inhibitor (intervention arm), and five with 3488 patients in the monoclonal antibodies (intervention arm). All 18 RCTs employed the same treatment arm, docetaxel.
A forest plot was used to summarize the pooled effect on OS of an antineoplastic agent [Figure 2], kinase inhibitors [Figure 3], and monoclonal antibodies [Figure 4], against docetaxel. The Hedge’s g values were the following: antineoplastic agents = 0.11; 95% CI: -0.03–0.26, kinase inhibitors = 0.04; 95% CI: -0.10–0.17, and monoclonal antibodies = 0.05; 95% CI: -0.02–0.13. The effect was low, the Hedge’s g being < 0.20. The meta-analysis indicates moderate heterogeneity in both kinase inhibitor (I2 = 42.0%) and antineoplastic agent therapy (I2 = 45.0%) and there is no heterogeneity in monoclonal antibodies (I2 = 0.0%). Docetaxel’s OS was slightly better than that of the antineoplastic agent, kinase inhibitors, and monoclonal antibodies due to the existence of moderate heterogeneity and less impact. As a result, the forest plot clearly showed that docetaxel’s OS was superior; yet the effect was minor.
The publication bias was determined using Egger’s test. Egger’s test yielded a p-value of 0.9479 [Figure 5], indicating that there was no publication bias.

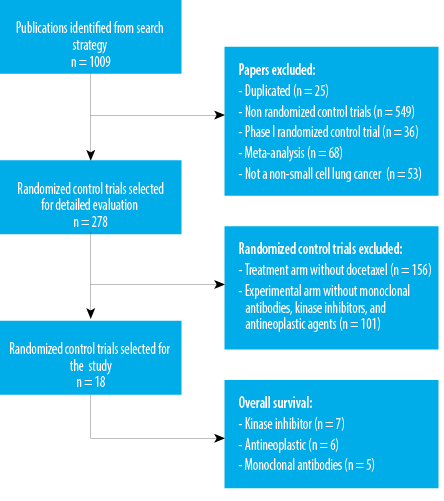 Figure 1: The flowchart summarizing the steps of the study selection.
Figure 1: The flowchart summarizing the steps of the study selection.
Table 1: Characteristics of the selected randomized controlled trials for meta-analysis.
|
1
|
Barlesi et al,13
2018
|
792
|
3
|
Avelumab 10 mg/kg/2W
|
Docetaxel 75 mg/m2/3W
|
5
|
|
2
|
Fehrenbacher et al,14
2016
|
287
|
1
|
Atezolizumab
1200 mg/3W
|
Docetaxel 75 mg/m2/3W
|
5
|
|
3
|
Garassino et al,15 2013
|
219
|
2
|
Erlotinib 150 mg/D
|
Docetaxel 75 mg/m2/3W
|
6
|
|
4
|
Garon et al,16
2014
|
1253
|
3
|
Ramucirumab 10 mg/kg/3W + Docetaxel
75 mg/m2/3W
|
Placebo + Docetaxel
75 mg/m2/3W
|
6
|
|
5
|
Gerber et al,17
2018
|
597
|
3
|
Bavituximab 3 mg/kg/W + Docetaxel
75 mg/m2/3W
|
Placebo + Docetaxel
75 mg/m2/3W
|
6
|
|
6
|
Herbst et al,18
2015
|
689
|
3
|
Pembrolizumab 10 mg/kg/3W
|
Docetaxel 75 mg/m2/3W
|
7
|
|
7
|
Jänne et al,19
2017
|
510
|
2
|
Selumetinib 75 mg/0.5D + Docetaxel 75 mg/m2/3W
|
Placebo + Docetaxel
75 mg/m2/3W
|
5
|
|
8
|
Kawaguchi et al,20 2014
|
301
|
2
|
Erlotinib 150 mg/D
|
Docetaxel 75 mg/m2/3W
|
5
|
|
9
|
Kubota et al,21
2015
|
596
|
1
|
S-1 80 mg/m2/D + cisplatin 60 mg/m2/W
|
Docetaxel 60 mg/m2/3W + Cisplatin 80 mg/m2/3W
|
6
|
|
10
|
Lee et al,22
2010
|
161
|
2
|
Gefitinib 250 mg/D
|
Docetaxel 75 mg/m2/3W
|
7
|
|
11
|
Manegold et al,23 2013
|
70
|
1
|
Cilengitide 600 mg/m2/0.5D
|
Docetaxel 75 mg/m2/3W
|
5
|
|
12
|
Ramlau et al,24
2012
|
913
|
2
|
(Ziv-)aflibercept 6 mg/kg/3W + Docetaxel 75 mg/m2/3W
|
Placebo + Docetaxel
75 mg/m2/3w
|
6
|
|
13
|
Reck et al,8
2014
|
1314
|
2
|
Docetaxel 75 mg/m2/3W + Nintedanib 200 mg/0.5D
|
Docetaxel 75 mg/m2/3W
|
6
|
|
14
|
Rittmeyer et al,25 2016
|
850
|
1
|
Atezolizumab 1200 mg/3W
|
Docetaxel 75 mg/m2/3W
|
7
|
|
15
|
Rodrigues-Pereira et al,26
2011
|
211
|
1
|
Pemetrexed 500 mg/m2/3W + Carboplatin
5 mg/mL/min
|
Docetaxel 75 mg/m2/3W + Carboplatin 5 mg/mL/min
|
5
|
|
16
|
Socinski et al,27
2010
|
146
|
1
|
Pemetrexed 500 mg/m2/3W + Carboplatin 6 mg/mL/min
|
Docetaxel 75 mg/m2/3W + Carboplatin 6 mg/mL/min
|
6
|
|
17
|
Yoh et al,28
2016
|
157
|
3
|
Ramucirumab 10 mg/kg/3W + Docetaxel 60 mg/m2/3W
|
Placebo + Docetaxel
75 mg/m2/3W
|
5
|
Drug class of intervention: 1: antineoplastic agent; 2: kinase inhibitors; 3: monoclonal antibodies; Treatment and dosage: W: week; D: day.

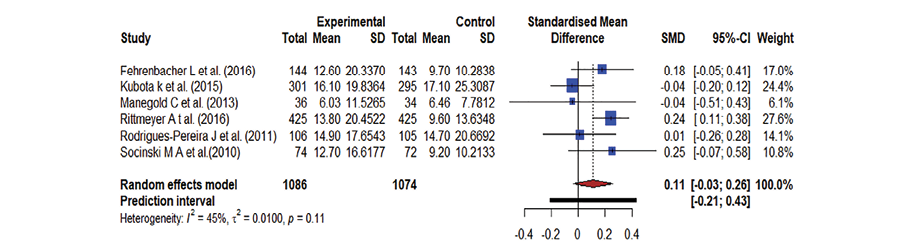 Figure 2: A forest plot representing the overall survival where docetaxel was used against antineoplastic agents; the Hedge’s corrected standardized mean difference (SMD) is 0.11 and Higgin’s and Thompson’s (I2) statistic is 45.0%.
Figure 2: A forest plot representing the overall survival where docetaxel was used against antineoplastic agents; the Hedge’s corrected standardized mean difference (SMD) is 0.11 and Higgin’s and Thompson’s (I2) statistic is 45.0%.

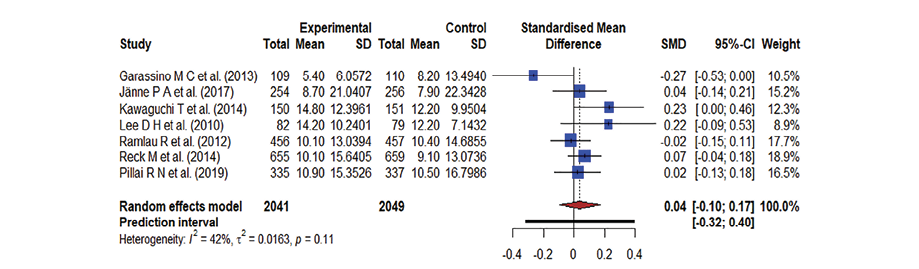 Figure 3: A forest plot representing the overall survival in docetaxel versus kinase inhibitors treatment; the Hedge’s corrected standardized mean difference (SMD) is 0.04 and Higgin’s and Thompson’s (I2) statistic is 42.0%.
Figure 3: A forest plot representing the overall survival in docetaxel versus kinase inhibitors treatment; the Hedge’s corrected standardized mean difference (SMD) is 0.04 and Higgin’s and Thompson’s (I2) statistic is 42.0%.

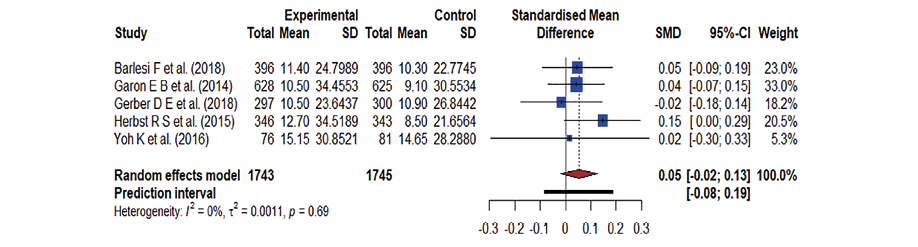 Figure 4: A forest plot representing the overall survival in docetaxel versus monoclonal antibodies treatment; the Hedge’s corrected standardized mean difference (SMD) is 0.05 and Higgin’s and Thompson’s (I2) statistic is 0.0%.
Figure 4: A forest plot representing the overall survival in docetaxel versus monoclonal antibodies treatment; the Hedge’s corrected standardized mean difference (SMD) is 0.05 and Higgin’s and Thompson’s (I2) statistic is 0.0%.

 Figure 5: A funnel plot showing publication bias. Each dot shape indicates one of the 18 studies.
Figure 5: A funnel plot showing publication bias. Each dot shape indicates one of the 18 studies.
Discussion
NSCLC is one of the most often diagnosed cancers and the major cause of cancer-related mortality worldwide. First-line treatment refers to the standard treatment for a diagnosed cancer. Second-line treatment is initiated where the first-line
treatment has failed or has intolerable adverse effects. For patients with advanced NSCLC, platinum-based dual-drug combination chemotherapy is the standard first-line treatment.21 For the second-line treatment, docetaxel is the only approved chemotherapy with evidence of improved survival and quality of life.23 Our primary goal was to compare the two chemotherapy regimens in terms of OS. Thus, our meta-analysis evaluated 18 RCTs comprising 9738 patients with stage III–IV NSCLC,13–29 to determine whether docetaxel as the second-line treatment improved their OS. The intervention group in each study received docetaxel-based chemotherapy. The corresponding control groups received either antineoplastic agents, kinase inhibitors, or monoclonal antibodies-
based chemotherapy.
Among the 18 clinical trials we reviewed, six trials conducted on 2160 patients compared docetaxel with antineoplastic agents. Kubota et al,21 compared S-1 plus cisplatin versus docetaxel plus cisplatin. Manegold et al,23 compared the improvement of OS between docetaxel and cilengitide. Rodrigues-Pereira et al,26 evaluated survival without toxicity in patients with advanced nonsquamous NSCLC treated with pemetrexed/carboplatin versus docetaxel/carboplatin as a first-line treatment. Fehrenbacher et al,14 and Rittmeyer et al,25 compared the safety and effectiveness of atezolizumab versus docetaxel in second- and third-line NSCLC. The permuted randomization method was used to receive either oral S-1 80 mg/m2/day plus cisplatin 60 mg/m2, cilengitide 600 mg/m2, pemetrexed 500 mg/m2/3W + carboplatin 5 mg/mL/min, atezolizumab 1200 mg or 60 mg/m2 docetaxel plus cisplatin 80 mg/m2, docetaxel 75 mg/m2, docetaxel 75 mg/m2/3W + carboplatin 5 mg/mL/min. The OS was the primary endpoint of all six studies. The increase in PD-L1 expression was related to improving OS rate. In individuals with previously treated NSCLC, oral S-1 plus cisplatin was not inferior to docetaxel plus cisplatin with hazard ratio (HR) = 1.013; 96.4% CI: 0.837–1.227.21 In the pemetrexed/carboplatin versus the docetaxel/carboplatin group, the OS was similar, with HR = 0.93; 95% CI: 0.66–1.32.26 Atezolizumab increased survival significantly with HR = 0.73; 95% CI: 0.53–0.99, p = 0.004 compared to docetaxel.14,21,23,25 For patients with advanced NSCLC and good performance status, current studies support platinum-based cytotoxic drug combinations as the first-line treatment. Although cisplatin may have a modest advantage in terms of survival or response, carboplatin is favored for combination chemotherapy in certain patients due to its good tolerability and convenience of administration.26
In patients with metastatic NSCLC, antibodies targeting the immune checkpoint molecules PD-1 or PD-L1 improve OS when compared to standard-of-care treatment.13 Five of the 18 studies compared docetaxel-based therapy with monoclonal antibodies-based therapy. The meta-analysis of a phase III trial of avelumab against docetaxel in patients with advanced NSCLC and disease progression after platinum-based chemotherapy is explained by Barlesi et al,13 and Garon et al;16 they compared the efficacy and safety of docetaxel with ramucirumab or placebo as second-line treatment for patients with stage IV NSCLC post-platinum-based therapy. The efficacy of bavituximab coupled with docetaxel in patients with previously treated advanced NSCLC is investigated by Gerber et al.17 Herbst et al,18 explained the efficacy and safety of pembrolizumab compared with docetaxel. Yoh et al,28 explained phase II, double-blind, randomized, placebo-controlled research in Japanese patients with NSCLC, which investigated the efficacy and safety of second-line ramucirumab-docetaxel. The patients were randomized to receive either avelumab 10 mg/kg, ramucirumab 10 mg/kg, docetaxel plus bavituximab 3 mg/kg, pembrolizumab 10 mg/kg or docetaxel 75 mg/m2, docetaxel plus placebo, docetaxel 60 mg/m2. OS was the main outcome in all the five studies which was assessed when several deaths in the PD-L1-positive sample had occurred. As compared to docetaxel, the median OS of avelumab showed a favorable safety profile, but did not enhance in the patients with platinum-treated PD-L1-positive NSCLC with an HR = 0.90; 95% CI: 0.72–1.12.13 Ramucirumab plus docetaxel increases survival in patients with stage IV NSCLC as a second-line treatment with HR = 0.86; 95% CI: 0.75−0.98, p = 0.023.16 In previously treated advanced NSCLC patients, the combination of bavituximab and docetaxel did not increase OS with HR = 1.06; 95% CI: 0.88–1.29, p = 0.533. OS was significantly greater for pembrolizumab 2 mg/kg versus docetaxel with HR = 0.71; 95% CI: 0.58–0.88, p = 0.0008.18 In patients with previously treated PD-L1-positive advanced NSCLC, pembrolizumab improved OS and had a favorable benefit-to-risk profile.28
For most patients who acquire the unresectable disease, palliative chemotherapy is the initial treatment approach.24 In a few patients, agents directing epidermal growth factor receptor ((EGFR); cetuximab), vascular endothelial growth factor ((VEGF); bevacizumab), or the EGFR tyrosine kinase pathway (erlotinib, gefitinib), heat shock protein 90 (HSP 90) may improve OS in addition to chemotherapy (bevacizumab, cetuximab) or administer as single agents, stabilizes oncogenic client proteins necessary for the survival, growth and invasive potential of cancer.24 This approach was taken by seven clinical studies which compared docetaxel with a kinase inhibitor. Tyrosine kinase inhibitors (TKIs) that target the EGFR tyrosine kinase are effective against NSCLC that has been previously treated. Lee et al,22 compared gefitinib to docetaxel in patients with advanced or metastatic NSCLC who had previously received platinum-based chemotherapy. Garassino et al,15 compared the efficacy of erlotinib to that of docetaxel, a common second-line treatment. In platinum pre-treated patients with advanced or metastatic non-squamous NSCLC, Ramlau et al,24 analyzed the efficacy of aflibercept (Ziv-aflibercept), a recombinant human fusion protein targeting the VEGF pathway, with or without docetaxel. Kawaguchi et al,20 compared the efficacy of erlotinib with docetaxel in previously treated patients with advanced NSCLC. Reck et al,8 evaluated the safety and efficacy of docetaxel with nintedanib as second-line therapy for NSCLC. For advanced Kirsten rat sarcoma virus (KRAS)-mutant NSCLC, Jänne et al,19 investigated the efficacy of the mitogen-activated protein kinase inhibitor, selumetinib and docetaxel with docetaxel alone as second-line therapy. Pillai et al,29 assessed the amalgamation of ganetespib and docetaxel for second-line therapy of patients with advanced lung adenocarcinoma. A randomized clinical method was used to receive either gefitinib 250 mg/d, erlotinib orally 150 mg/day, (Ziv-) aflibercept 6 mg/kg intravenous plus docetaxel 75 mg/m2, erlotinib 150 mg/D, nintedanib 200 mg orally, selumetinib 75 mg/0.5D + docetaxel 75 mg/m2/3W, ganetespib 150 mg/m + docetaxel 75 mg/m2, IV placebo plus docetaxel 75 mg/m2, or placebo + docetaxel 75 mg/m2/3W until unacceptable side effects or progression of disease based on histology, Eastern Cooperative Oncology Group (ECOG) performance status, earlier bevacizumab treatment, and presence of brain metastases. The OS was estimated as a primary and secondary endpoint in these studies. OS had longer improvement in gefitinib than docetaxel with HR = 0.870; 95% CI: 0.613–1.236, two-sided p = 0.437.15 The addition of (Ziv-) aflibercept to conventional docetaxel therapy has HR = 1.01; 95% CI: 0.87–1.17 and did not improve OS.24 Erlotinib was unsuccessful in showing an improvement in OS when compared to docetaxel in an EGFR with HR = 0.91; 95% CI: 0.68–1.22, p = 0.53.22 Nintedanib in combination with docetaxel improves the OS compared to docetaxel plus placebo with HR = 0.75; 95% CI: 0.60–0.92, p = 0.0073. Addition of selumetinib to docetaxel with HR = 1.05; 95% CI: 0.85–1.30, p = 0.64 did not improve OS compared to docetaxel.19 The addition of ganetespib to docetaxel did not increase survival in patients with advanced-stage lung adenocarcinoma receiving salvage therapy.29
There are certain limitations in the current study that must be kept in mind while evaluating its results. First, the different treatment schedules contributed to increased clinical heterogeneity in the meta-analysis, making the interpretation more difficult. In three trials, docetaxel was used in conjunction with other medicines, either cisplatin or carboplatin, in the control arm. The quality of our results also depended on the quality of findings of each of the 18 studies we investigated. Based on the analysis of these clinical trials, the findings must be interpreted with caution when applying to other populations — such as in South Asia and the Middle East. Since OS was the widely used outcome, we could conclude that this meta-analysis supports the docetaxel-based second-line therapy for patients who have advanced NSCLC in terms of outcome. The results also support docetaxel’s superiority to antineoplastic agents, kinase inhibitors, and monoclonal antibodies. Along with the parameters used in the study, biological behavior subgroups such as those entirely refractory, those with partial and incomplete responses, and those with short and extended disease-free intervals need to be examined in future meta-analysis investigations.
Conclusion
In a total of 9738 patients from 18 studies, the OS improved in patients who received docetaxel-based therapy as second-line treatment for advanced NSCLC than in those who received antineoplastic agents, kinase inhibitors, and monoclonal antibodies-based treatment. In the overall meta-analysis, patients in the standard treatment arm had a slightly better OS than those in the intervention treatment arm. It is a meta-analysis of clinical trials conducted before the era of immunotherapy and targeted therapy. We may infer that docetaxel-based second-line therapy for patients with advanced NSCLC is supported by this meta-analysis. As per our results, second-line treatment with docetaxel is more effective to enhance the OS of patients with advanced NSCLC compared to antineoplastic agents, monoclonal antibodies, and kinase inhibitors.
Acknowledgments
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Lung cancer: overview & facts. WebMD2021. [cited 2021 Oct 15]. Available from: https://www.webmd.com/lung-cancer/guide/lung-cancer-overview-facts.
- 2. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018 Jan;553(7689):446-454.
- 3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021 May;71(3):209-249.
- 4. Jain AS, Prasad A, Pradeep S, Dharmashekar C, Achar RR, Ekaterina S, et al. Everything old is new again: drug repurposing approach for non-small cell lung cancer targeting MAPK signaling pathway. Front Oncol 2021 Oct;11:741326.
- 5. Jiang J, Liang X, Zhou X, Huang R, Chu Z. A meta-analysis of randomized controlled trials comparing carboplatin-based to cisplatin-based chemotherapy in advanced non-small cell lung cancer. Lung Cancer 2007 Sep;57(3):348-358.
- 6. Rosero ID, Ramírez-Vélez R, Lucia A, Martínez-Velilla N, Santos-Lozano A, Valenzuela PL, et al. Systematic review and meta-analysis of randomized, controlled trials on preoperative physical exercise interventions in patients with non-small-cell lung cancer. Cancers (Basel) 2019 Jul;11(7):944.
- 7. Xiao W, Hong M. Concurrent vs sequential chemoradiotherapy for patients with advanced non-small-cell lung cancer: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2021 Mar;100(11):e21455.
- 8. Reck M, Kaiser R, Mellemgaard A, Douillard J-Y, Orlov S, Krzakowski M, et al; LUME-Lung 1 Study Group. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014 Feb;15(2):143-155.
- 9. Docetaxel in treating patients with non-small cell lung cancer - full text view - clinicaltrials.gov. Clinicaltrials.gov2021. [cited 2021 Oct 15]. Available from: https://clinicaltrials.gov/ct2/show/NCT00022022.
- 10. NCI dictionary of cancer terms. National Cancer Institute2021 [cited 2021 Oct 15]. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/overall-survival.
- 11. Viney RC, Boyer MJ, King MT, Kenny PM, Pollicino CA, McLean JM, et al. Randomized controlled trial of the role of positron emission tomography in the management of stage I and II non-small-cell lung cancer. J Clin Oncol 2004 Jun;22(12):2357-2362.
- 12. Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2018 Aug;97(33):e11936.
- 13. Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 2018 Nov;19(11):1468-1479.
- 14. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al; POPLAR Study Group. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016 Apr;387(10030):1837-1846.
- 15. Garassino MC, Martelli O, Broggini M, Farina G, Veronese S, Rulli E, et al; TAILOR trialists. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013 Sep;14(10):981-988.
- 16. Garon EB, Ciuleanu T-E, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014 Aug;384(9944):665-673.
- 17. Gerber DE, Horn L, Boyer M, Sanborn R, Natale R, Palmero R, et al. Randomized phase III study of docetaxel plus bavituximab in previously treated advanced non-squamous non-small-cell lung cancer. Ann Oncol 2018 Jul;29(7):1548-1553.
- 18. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016 Apr;387(10027):1540-1550.
- 19. Jänne PA, van den Heuvel MM, Barlesi F, Cobo M, Mazieres J, Crinò L, et al. Selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRAS-mutant advanced non–small cell lung cancer: the SELECT-1 randomized clinical trial. JAMA 2017 May;317(18):1844-1853.
- 20. Kawaguchi T, Ando M, Asami K, Okano Y, Fukuda M, Nakagawa H, et al. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: docetaxel and erlotinib lung cancer trial (DELTA). J Clin Oncol 2014 Jun;32(18):1902-1908.
- 21. Kubota K, Sakai H, Katakami N, Nishio M, Inoue A, Okamoto H, et al; Tokyo Cooperative Oncology Group. A randomized phase III trial of oral S-1 plus cisplatin versus docetaxel plus cisplatin in Japanese patients with advanced non-small-cell lung cancer: TCOG0701 CATS trial. Ann Oncol 2015 Jul;26(7):1401-1408.
- 22. Lee DH, Park K, Kim JH, Lee J-S, Shin SW, Kang J-H, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res 2010 Feb;16(4):1307-1314.
- 23. Manegold C, Vansteenkiste J, Cardenal F, Schuette W, Woll PJ, Ulsperger E, et al. Randomized phase II study of three doses of the integrin inhibitor cilengitide versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer. Invest New Drugs 2013 Feb;31(1):175-182.
- 24. Ramlau R, Gorbunova V, Ciuleanu TE, Novello S, Ozguroglu M, Goksel T, et al. Aflibercept and docetaxel versus docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol 2012 Oct;30(29):3640-3647.
- 25. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al; OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017 Jan;389(10066):255-265.
- 26. Rodrigues-Pereira J, Kim J-H, Magallanes M, Lee DH, Wang J, Ganju V, et al. A randomized phase 3 trial comparing pemetrexed/carboplatin and docetaxel/carboplatin as first-line treatment for advanced, nonsquamous non-small cell lung cancer. J Thorac Oncol 2011 Nov;6(11):1907-1914.
- 27. Socinski MA, Manikhas GM, Stroyakovsky DL, Makhson AN, Cheporov SV, Orlov SV, et al. A dose finding study of weekly and every-3-week nab-Paclitaxel followed by carboplatin as first-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol 2010 Jun;5(6):852-861.
- 28. Yoh K, Hosomi Y, Kasahara K, Yamada K, Takahashi T, Yamamoto N, et al. A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy. Lung Cancer 2016 Sep;99:186-193.
- 29. Pillai RN, Fennell DA, Kovcin V, Ciuleanu T-E, Ramlau R, Kowalski D, et al. Randomized phase III study of ganetespib, a heat shock protein 90 inhibitor, with docetaxel versus docetaxel in advanced non-small-cell lung cancer (GALAXY-2). J Clin Oncol 2020 Feb;38(6):613-622.