Chronic hepatitis B (CHB) infection is a major global health problem worldwide, with an estimated prevalence of 3.9%, corresponding to approximately 292 million infected people.1 Hepatitis B virus (HBV) can cause acute and chronic liver disease, including cirrhosis and hepatocellular carcinoma (HCC). Worldwide, CHB is responsible for 60–80% of the cases of HCC.2 In 2015, an estimated 887 000 deaths worldwide occurred due to HBV infection.3 There are no clear data about the true prevalence of CHB in Oman; however, it is estimated to be an intermediate prevalence (2–7%).4,5
HBV is mainly transmitted via blood and body fluids. The risk of transmitting the virus depends mainly on the viral load.6 HBV transmission risk factors show substantial variation globally. Vertical transmission is the most common mode of transmission in high endemicity regions like South Asia, which is responsible for most cases of CHB. Intermediate endemicity of hepatitis B in areas such as the Middle East is attributed to early horizontal transmission of the virus in preschool-age children.7 Other common modes of transmission (late horizontal transmission) include unsafe medical settings, sexual contact (heterosexual or homosexual), and intravenous drug use (IDU).8 Less common risk factors contributing to the transmission of HBV include shaving in barbershop, tattooing, piercing, and acupuncture. The significance of these practices in spreading the disease has been minimally investigated. Different studies from the Middle East showed that shaving in barbershops could be a risk for HBV transmission.9–12 In a cross-sectional study in Iran, tattooing was found to be an independent risk factor for being chronically infected with HBV.13 A recent study from Oman reported a positive family history of HBV, traditional cautery (wasm), body piercing, surgery, and blood transfusion in 70%, 65%, 40%, 18.2%, and 4.5% of patients, respectively.5
Certain risk factors for HBV transmission are identified, including hepatitis B surface antigen (HBsAg) and e-antigen (HBeAg) positivity, and high maternal HBV viral load.
Research to determine the major risk factors for transmitting HBV in Oman has been lacking. Therefore, this study was conducted to determine the prevalence of major risk factors for acquiring HBV among Omani patients positive for HBsAg. As there has not been a control group in this study, it is not possible to assess the causative role of risk factors for HBV acquisition in Oman. However, the results of this study will provide a further understanding of the epidemiology of HBV in Oman, and will aid in identifying people who are at higher risk of acquiring this infection.
Methods
All patients were recruited from adult hepatology clinics at Sultan Qaboos University Hospital and Armed Forces Hospital, Oman, from February 2009 to July 2013. Ethical approval was taken from the ethics committees in both hospitals. A trained interviewer interviewed the patients either through a face-to-face interview during their clinic visits or through a telephone interview. The study was explained to the patients, and consent was obtained before starting the interview. All Omani patients with positive HBsAg aged ≥ 13 years were included. Non-Omani patients and patients with incomplete questionnaires were excluded from the study.
Data was collected using a two-page questionnaire recording patients’ demographic characteristics such as age, sex, marital status, educational level, and occupation. Participants were also questioned about their immunization history and their frequency of exposure to identified and potential risk factors for HBV transmission before their date of diagnosis. Those risk factors included a history of hospitalization, major surgeries, organ transplantation, blood transfusion, endoscopy, hemodialysis, chemotherapy, dental visits, and contact with infected people.
The questionnaire was derived from known and suggested risk factors in international literature. Each interview took five to 15 minutes, and patients were given a chance to ask any questions before and after finishing the questionnaire.
Participants were grouped into three different age groups depending on the year of introduction of the HBV immunization program in Oman. The three age groups were: 13–22 for patients born after the introduction of HBV vaccine to Expanded Program on Immunization in August 1990; 23–28 for patients born before August 1990 but who presumably had completed the catch-up school vaccine campaigns in 2004–2005; and ≥ 29 for patients born before the introduction of HBV vaccination.
Risk factors for the transmission of HBV were grouped as nosocomial, family exposure, or high-risk behaviors. Nosocomial risk factors included a history of hospitalization, major surgery, organ transplantation, endoscopy, blood transfusion dialysis, and dental treatment. Family-related risk factors included family history of HBV, individuals currently living with HBV (later classified as sexual or non-sexual contact), mothers’ history of HBV, and family history of liver disease. High-risk behaviors included piercings, regular shaving with a barber, wasm (a traditional method of healing using cauterization), traditional phlebotomy, acupuncture, circumcision, and multiple sexual partners.
Participants were asked where piercing or circumcision was done, which was then classified as clinical and non-clinical settings.
The sample size required was determined by the number of patients interviewed during the data collection period. This was expected to be 300 patients. In a previous study of CHB carriers in Iran that looked at 560 patients, they found that endoscopy, major surgery, and tattooing were independent risk factors for CHB with percentages of 54.8%, 44.5%, and 8.5%, respectively.13 If the situation was assumed to be similar in Oman, a sample size of 300 participants would enable us to estimate the proportion tattooed with 95% confidence intervals (CIs) of approximately ±5%, and the proportion with endoscopy or surgery with a 95% CI of approximately ±6%.
Epi info version 7 was used to calculate the frequency of HBV transmission risk factors in the total studied population. The risk factor frequency was stratified by age groups (< 13, 13–23, > 28), sex (male and female), and educational level (pre-secondary, secondary, and post-secondary). A chi-square test was performed to examine the association between risk factor frequency and sex, age, or educational level and reported when significant. A p-value of < 0.050 was considered significant.
Results
Out of 365 HBV-positive patients eligible to participate in this study, 274 were included in the final analysis. A total of 91 patients were excluded due to inability to contact and incomplete questionnaire. The demographic features are summarized in Table 1. The number of male patients was 143, representing 52.2% of the entire cohort. The majority of the participants came from the
Al Batinah (32.1%), A'Dakhiliyah (25.9%), Muscat (19.0%), and A'Sharqiyah (16.4%) governorates. Most participants (77.7%) had a secondary school and above level of education. A minority of participants (3.3%) worked in high-risk jobs, including three nurses, two police officers, one doctor, one medical student, one medical assistant, and one medical orderly. The median age for men was 35.9 years and 35.1 years for women, with 75.5% aged 20–39 [Figure 1].
Table 1: Demographic characteristics of the participants (n = 274).
|
Age, median (range), years |
35.5 (19–86) |
|
|
Gender |
|
|
|
Male |
143 |
52.2 |
|
Female |
131 |
47.8 |
|
Marital status |
|
|
|
Married |
244 |
89.1 |
|
Not married |
30 |
10.9 |
|
Governorates |
|
|
|
Al Batinah |
88 |
32.1 |
|
A'Dakhiliyah |
71 |
25.9 |
|
Muscat |
52 |
19.0 |
|
A'Sharqiyah |
45 |
16.4 |
|
Others |
18 |
6.6 |
|
Education Level |
|
|
|
Secondary school and above |
213 |
77.7 |
|
Occupation |
|
|
|
Non-high-risk occupations |
264 |
96.4 |
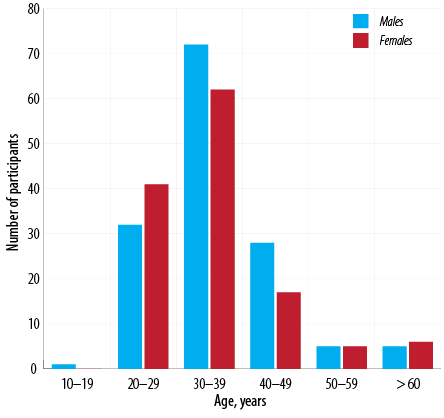
Figure 1: Age distribution of participants (n = 274).
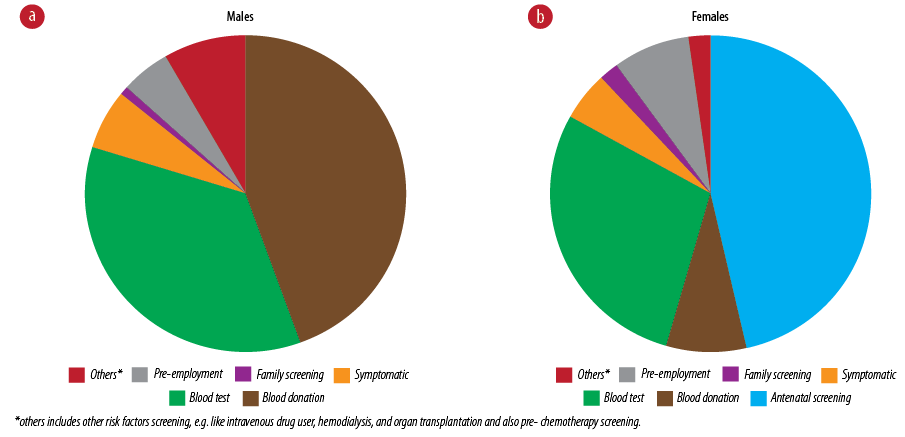
Figure 2: (a) Means of diagnosing hepatitis B virus in male (n = 143) and (b) female patients (n = 131).
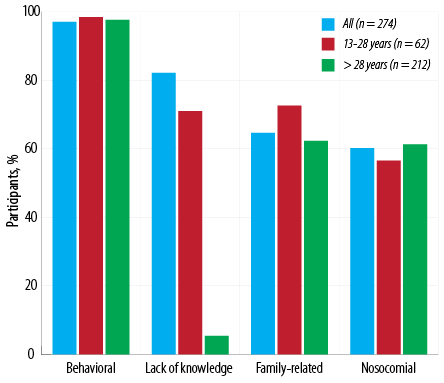
Figure 3: Prevalence of hepatitis B virus transmission risk factors in this study group (n = 274).
Table 2: Frequency of nosocomial risk factors by sex, age, and educational level (n = 274).
|
Surgery |
81 (29.6) |
37 (25.9) |
44 (33.6) |
4 (66.7) |
16 (28.6) |
61 (28.8) |
21 (34.4) |
36 (32.1) |
24 (23.8) |
|
Hospitalization |
77 (28.1) |
42 (29.4) |
35 (26.7) |
3 (50.0) |
13 (23.2) |
61 (28.8) |
18 (29.5) |
25 (22.3) |
34 (33.7) |
|
Blood transfusion |
24 (8.8) |
13 (9.1) |
11 (8.4) |
0 (0.0) |
5 (8.9) |
19 (8.9) |
7 (11.5) |
12 (10.7) |
5 (5.0) |
*The frequency of endoscopy shows statistical difference between age groups, x2 = 7.8227, df = 2, p = 0.020.
Data were given as n (%).
Table 3: Frequency of family-related risk factors by sex, age, and educational level (n = 274).
|
Maternal history of HBV |
|
|
|
* |
|
|
|
|
|
|
Yes |
24 (8.8) |
8 (5.6) |
16 (12.2) |
2 (33.3) |
8 (14.3) |
14 (6.6) |
2 (3.3) |
13 (11.6) |
9 (8.9) |
|
No |
100 (36.5) |
52 (36.4) |
48 (36.6) |
3 (50.0) |
18 (32.1) |
79 (37.3) |
22 (36.1) |
39 (34.8) |
39 (38.6) |
|
Unknown |
150 (54.7) |
83 (58.0) |
67 (51.2) |
1 (16.7) |
30 (53.6) |
119 (56.1) |
37 (60.7) |
60 (53.6) |
53 (52.5) |
|
Family history of HBV |
|
** |
|
|
|
|
** |
|
|
|
Yes |
150 (54.7) |
67 (46.8) |
83 (63.4) |
4 (66.7) |
36 (64.3) |
101 (47.6) |
19 (31.1) |
65 (58.0) |
66 (65.3) |
|
No |
61 (22.3) |
35 (24.5) |
26 (19.8) |
1 (16.7) |
8 (14.3) |
52 (24.5) |
21 (34.4) |
24 (21.4) |
16 (15.8) |
|
Unknown |
63 (23.0) |
41 (28.7) |
22 (16.8) |
1 (16.7) |
12 (21.4) |
50 (23.6) |
21 (34.4) |
23 (20.5) |
19 (18.8) |
|
Contact with HBV infected person |
|
*** |
|
|
|
|
*** |
|
|
|
Sexual contact |
31 (11.3) |
11 (7.7) |
20 (15.3) |
1(16.7) |
8 (14.3) |
22 (10.4) |
4 (6.6) |
16 (14.3) |
11 (10.9) |
|
Non-sexual contact |
117 (42.7) |
54 (37.8) |
63 (48.1) |
2(33.3) |
27 (4 8.2) |
88 (41.5) |
16 (26.2) |
51 (45.5) |
50 (49.5) |
|
No contact |
95 (34.7) |
58 (40.6) |
37 (28.2) |
2(33.3) |
12 (21.4) |
81 (38.2) |
28 (45.9) |
35 (31.3) |
32 (31.7) |
|
Unknown |
31 (11.3) |
20 (21.0) |
11 (8.4) |
1(16.7) |
9 (16.1) |
21 (9.9) |
13 (21.3) |
10 (8.9) |
8 (7.9) |
|
Family history of liver disease |
|
|
|
|
|
|
|
|
|
|
Yes |
27 (9.9) |
13 (9.1) |
14 (10.7) |
2 (33.3) |
8 (14.3) |
17 (8.0) |
4 (6.6) |
10 (8.9) |
13 (12.9) |
|
No |
199 (72.6) |
102 (71.3) |
97 (74.0) |
3 (50.0) |
36 (64.3) |
160 (75.5) |
41 (67.2) |
85 (75.9) |
73 (72.3) |
HBV: hepatitis B virus.
*Significant difference in mother’s history of HBV infection by age (x2 = 9.5164, df = 4, p = 0.049).
**Significant difference in family history of hepatitis B by gender (x2= 9.0944, df = 3, p = 0.016) and educational level (x2 = 20.9362, df = 6, p = 0.002).
***Living with HBV infected persons significantly differed between the three educational level groups (x2 = 19.1856, d.f. = 6, p = 0.009).
Data were presented as n (%).
Table 4: Frequency of high-risk behaviors by gender, age, and educational level (n = 274).
|
Lack of knowledge of HBV infection |
225 (82.1) |
119 (83.2) |
106 (80.9) |
4 (66.7)* |
40 (71.4) |
181 (85.4) |
60 (98.4)* |
90 (80.4) |
70 (69.3) |
|
Piercing in non-clinical settings |
128 (46.7) |
15 (10.5) |
113 (86.3) |
3 (50.0) |
21 (37.5) |
104 (49.1) |
33 (54.1) |
48 (42.9) |
47 (46.5) |
|
Regular shaving with a barber |
128 (46.7) |
128 (89.5) |
0 (0.0) |
2 (33.3) |
22 (39.3) |
104 (49.1) |
25 (41.0) |
54 (48.2) |
49 (48.5) |
|
Traditional cautery (wasm#)** |
132 (49.6) |
75 (52.4) |
61 (46.6) |
1 (16.7) |
21 (37.5) |
114 (53.8) |
39 (63.9) |
55 (49.1) |
42 (41.6) |
|
Traditional phlebotomy |
14 (5.1) |
6 (4.2) |
8 (6.1) |
1 (16.7) |
3 (5.4) |
10 (4.7) |
5 (8.2) |
5 (4.5) |
4 (4.0) |
|
Acupuncture |
4 (1.5) |
1 (0.7) |
3 (2.3) |
0 (0.0) |
0 (0.0) |
4 (1.9) |
2 (3.3) |
0 (0.0) |
2 (2.0) |
|
Circumcision in non-clinical settings |
37 (13.5) |
37 (25.9) |
0 (0.0) |
0 (0.0) |
2 (3.6) |
35 (16.5) |
12 (19.7)*** |
16 (14.3) |
9 (8.9) |
|
Extramarital sexual contact |
24 (8.8) |
24(16.8%)**** |
0 (0) |
1 (16.7) |
2 (3.6) |
21 (9.9) |
6 (9.8) |
11 (9.8) |
7 (6.9) |
#An alternative medical practice used to treat jaundice by applying a hot metal implement to the skin.
*Significant difference in hepatitis B awareness by age and educational level (x2 = 6.8664, df = 2, p = 0.030 and x2= 22.8042, df = 2, p < 0.001, respectively).
** Significant difference in wasm by age (x2 = 10.5833, df = 4, p = 0.025) and educational level (x2= 10.5833, df = 4, p = 0.025 and x2= 10.5394, df = 4, p = 0.030).
***Significant difference in circumcision between the three educational groups (x2 = 13.0917, df. = 6, p = 0.040).
****Significant difference in extramarital sexual contact by sex (Fisher's exact test, p < 0.001).
HBV: hepatitis B virus; IDU: intravenous drug abuse.
Blood donation was the most common means by which HBV was diagnosed in males (45.0%), while antenatal screening was the most common in females (46.0%) [Figure 2]. Blood test for other reasons (e.g., after birth, before or after surgery, multi-transfusion screen, health check-up) was the second most common source of diagnosis in males and females, 35.0% and 29.0%, respectively. The least common means of diagnosis in both sexes was family screening.
Figure 3 summarizes the prevalence of HBV transmission risk factors grouped as behavioral risk factors, perinatal and household contact, or nosocomial risk factors. High-risk behaviors were very common in this study population with similar distribution in the two age groups. Almost two-thirds of the participants had a history of perinatal and household contact (family-related) transmission risks, which were more common in those under 28 years old. Around 60.0% of the interviewees had at least a history of nosocomial risks. Only one participant reported no history of HBV acquisition risk factors. Concerning HBV transmission risk factors, intra-familial contact with HBV infected persons and behavioral risks, such as body piercing (females) and barber shaving (males), were more common than nosocomial risk factors.
The frequency of nosocomial risk in this study population appears to be low [Table 2]. The majority of participants had no history of surgery (70.4%), hospitalization (71.9%), blood transfusion (91.2%), and endoscopy (93.4%). Only one patient had a history of dialysis, and none of the participants had a history of organ transplantation. With further statistical analysis, a significant difference in the frequency of endoscopy between age groups was noted, but the number of participants in the 13–28 years was smaller than in the two remaining age groups. The chi-square test did not show any significant difference in the frequency of other nosocomial risk factors between sex, age, and educational level.
Assessment of the effect of perinatal, early horizontal, and spousal transmission of HBV among participants [Table 3] revealed that more than half of the participants had a positive family history of HBV infection. This was significantly different between the sexes (p = 0.016) and educational level (p = 0.002). A significant difference was also found between the three age groups (< 13, 13–23, > 28) of HBV positive mothers (33.3% 14.3%, and 6.6%, respectively; p = 0.049). Contact with HBV-infected persons revealed a statistically significant difference among different educational levels (p = 0.009).
Table 4 shows the prevalence of high-risk behaviors in the participants. A minority of participants indicated history of tattoos (0.4%), acupuncture (1.5%), traditional phlebotomy (5.1%), extramarital sexual contact (8.8%), and circumcision in non-clinical settings (13.5%). The frequency of circumcision in non-clinical settings decreased significantly with increasing educational levels (p = 0.040). Unawareness about HBV infection (82.1%), piercing in non-clinical settings (46.7%), regular shaving with a barber (46.7%), and wasm (49.6%) were more common in this studied group. There was a significant association between hepatitis B and age groups and educational levels (p =0.030 and p < 0.001, respectively). The relationship between having wasm by age, and educational level was also significant, p = 0.025 and p = 0.030, respectively. There was also a significant association between having an extramarital sexual relationship by sex (p < 0. 001).
Discussion
The HBV vaccine was introduced to the Expanded Program on Immunization in Oman in August 1990, aiming to reduce the prevalence of HBV carriage to below 2% in the general population.14 All newborns well enough to be discharged from the hospital (including premature and low weight infants) are indicated for the first HBV vaccine dose within the first 12 hours of life. Active immunization using the three-dose vaccine plus passive immunization by administering hepatitis B immunoglobulins to neonates born to HBsAg mother regardless of her HBeAg status is followed in Sultan Qaboos University Hospital and Armed Forces Hospital but not in Ministry of Health (MoH) hospitals.5 To increase the coverage rate of the HBV vaccination strategy, hepatitis B catch-up school campaigns were conducted from 2001–2004 to vaccinate school children who were born before August 1990.14
The MoH also implemented other strategies to improve the control of diseases in Oman.15 These include screening all family contacts of HBsAg positive patients, vaccinating HBsAg negative children below the age of 10 years, and screening all blood and blood products for viral hepatitis.
Certain risk factors for HBV transmission, such as the family history of hepatitis B, wasm, body piercings, and surgeries were common among a group of Omani patients.5 Our current study revealed similar findings, which demonstrated the common sources of identifying HBV infected patients.
The male to female ratio was almost equal to one. This finding is consistent with other studies conducted in Oman and Iran.5,16,17 On the other hand, men account for most cases of HBV in most parts of the world.4,7,8,12,18–20 It may be that Oman has different epidemiology of hepatitis B, or this might result from referral bias. Almost three-quarters of the participants lie in the 20–39 age group. Generally, this would contribute indirectly to the economic burden associated with job loss, reduced work productivity, and premature death.21 Specifically, most female patients were of childbearing age, which maintains the risk of mother to child transmission of hepatitis B.
Iatrogenic risk factors seemed to be the least frequent among our participants. Almost one-third of participants have a history of surgery, which was the most prevalent nosocomial risk factor. A case control study from Iran, which has a similar hepatitis B prevalence to Oman, found that a history of surgery is an independent risk factor for CHB carriage.13 Moreover, the major sources of HBV infection in Bahrain and Yemen were hospital-acquired infections, including dental procedures, surgical operations, and blood transfusions.22,23 On the contrary, studies conducted in other Arab and African countries showed an insignificant role of surgical interventions in transmitting HBV.24 Therefore, it is difficult to assess the real impact of surgical interventions in transmitting hepatitis B. Such mode of transmission could be significantly reduced by having high standards for sterilization, disinfection, screening, and training.
Intra-familial transmission of hepatitis B can be through vertical or horizontal mode by either sexual or non-sexual contact. The latter is thought to be the predominant mode of hepatitis B transmission in the Middle East.7 In the current sample, only 8.8% of participants reported positive mother status for HBV, which was significantly associated with the age of participants (p < 0.050), while more than half of the participants reported familial contact with HBV positive persons (11.3% sexual contact vs. 42.7% non-sexual contact).
The majority of the participants who reported sexual contact also have siblings with HBV. This suggests the possibility of the infection occurring earlier in life. HBV is a highly infective virus, and HBeAg is most prevalent in children, which is associated with a high infectivity rate.7
Multiple studies showed shared use of contaminated materials such as razors, toothbrushes, towels, eating utensils may account for early horizontal transmission of HBV among family members,7,12,25 while the availability of sanitization tools within the household is reported to be protective against transmission.26
Our study revealed a significant association between high educational level and contact with an HBV-positive person (p < 0.050). This finding differs from the previous reports of Zhang et al,27 and Merat et al,17 where lower educational level and income are identified risk factors for HBsAg positivity in both high and intermediate endemicity regions. This might be since highly educated persons are more likely to convince their family members to screen for hepatitis B and, therefore, higher reporting level.
From this study, high-risk behaviors were noted to be common in this group of patients. The majority of females had body piercing in non-clinical settings, and a similar proportion of males shaved regularly with barbers. Determining the role of these practices in transmitting HBV is difficult as it is poorly researched within the region. Although body piercing has been identified as a potential risk factor for HBV infection,27,28 a recent study in the Netherlands found that body piercings did not increase the risk of HBV infection for the Dutch population.29 While this may be true for the Netherlands, where HBV endemicity is low and hygiene guidelines have been introduced in piercing shops, in Oman HBV is more endemic, and most of our participants had their piercings at home. Moreover, piercings for females are usually done at a young age in Oman where the risk of chronic carriage is higher.30
Studies from the Middle East showed barbers had low to moderate awareness that contaminated razors can transmit hepatitis, and 46% of shaves were done with reused razors.9,10 HBV DNA was detected in 6.6% of used razor blades, and cuts from barbershops are associated with HBV transmission with an odds ratio of 4.74.11,12 Despite the effort of the Omani government to ensure the safety of these practices by requiring the use of one-time disposable razors, the availability of sanitization tools in all barbershops with regular inspection, their contribution to HBV transmission cannot be ruled out entirely.
None of the participants in our study reported a history of IDU, and only 16.8% reported multiple sexual partners. Such risk factors are the most common modes of HBV transmission in lower endemicity regions in Western societies. In the USA, sexual contact (heterosexual or homosexual) and IDU account for 40.9% and 18.2% of acute hepatitis B, respectively.31 In China, on the other hand, where HBV is of high endemicity, no association between acute hepatitis B and sexual contact or IDU was found in univariate and multivariate regression analyses.28 The reason for this may be a similar attitude of Chinese and Omani people towards these behaviors. Studies in the Middle East reported that HBV prevalence among IDU ranges between 6% to 44.3%.9,13
Antenatal screening seems to be one of the most effective detection strategies for women in our sample; almost half of female participants were discovered to be HBsAg positive during pregnancy. Despite the evidence of the higher prevalence of HBV infection among Omani pregnant women (7.1%) compared to those in other Gulf Cooperation Council states, (Saudi Arabia 1.6%, UAE 1.5%, and Qatar 1%),24,32 routine antenatal screening for HBsAg is not available at MoH institutes. With the introduction of neonatal vaccination in 1990, it would take around 20–40 years for the vaccine alone to eliminate vertical transmission of HBV in Oman. The Center for Disease Control (CDC) recommends routine screening of all pregnant women for HBsAg and the administration of active and passive vaccination for infants born to HBsAg-positive mothers.33 These measures are associated with 90–100% effectiveness in preventing the transmission of the virus in neonates born to mothers with HBV infection34 and should be considered in Oman. However, in mothers positive for HBeAg with high viral load, post prophylaxis failure is possible, and the introduction of antiviral therapy within the third trimester should be considered.35
Despite the evidence of common contact with HBV-positive family members among the participants, only 1.5% were diagnosed through contact screening. This suggests that contact screening is not being widely applied. Greater focus on contact screening could aid in the control of HBV infection in Oman by identifying non-immune contacts and vaccinating them. Furthermore, it could act as a secondary prevention measure by identifying those with chronic infection, providing them with the appropriate treatment or follow-up, and reduce the long-term complications (cirrhosis or HCC) and mortality associated with chronic infection.
The majority of our participants did not know about HBV infection before their diagnosis. Educating individuals about hepatitis B risk factors could help to reduce the risk of spreading the virus. It has been shown that improving awareness regarding risk factors of HBV transmission has led to a decrease in HBV prevalence in Iran.36
There are some strengths and weaknesses in this study. One of the strengths is the use of a standard questionnaire containing closed questions throughout the study. The same interviewer filled all the questionnaires during a direct interview with the participants for consistency. This way, any misinterpretations of questions by patients were corrected at the time of the interview thus minimizing any false reporting. Moreover, any potential heterogeneity in reporting that may have arisen from using two interviewing methods (face-to-face vs. telephone) would be minimized. Another strength is that both hospitals screen pregnant women for HBsAg, giving a more accurate representation of hepatitis B distribution between the sexes.
The limitations of this study might arise from referral bias and recall error. Referral bias from recruiting patients from tertiary referral hospitals could play a role in this study. Patients under the age of 13 years are not seen at the outpatient clinics where patients were recruited for this study. This would limit our investigations regarding the vertical transmission of HBV and would make the findings of this study only applicable to adults in Oman. Also, as both hospitals are located in Muscat, patients from regions outside Muscat might be underrepresented. This was noted for Dhofari patients. Only 0.7% of our participants came from Dhofar, while Communicable diseases Surveillance and Control (CDSC), Ministry of Health, Oman reports show that the Dhofar governorate accounted for almost a quarter of the cumulative incidence of acute hepatitis B infection from 1991 to 2010.37 Risk factors for HBV transmission might differ in Dhofari patients compared to the general population.
In any study in which data is collected during an interview with the patients, recall error is expected to occur, especially in patients with chronic infection, patients diagnosed with the disease a long time ago, and older patients with a long history. Patients might report that an exposure (risk factor) preceded the outcome (hepatitis B) even if it occurred after; hence, overestimating the role of these risk factors in this group of patients. Using the date of diagnosis as the reference date helped to minimize this recall error. In addition, risk factors such as sexual activity or intravenous drug use are difficult to investigate and maybe underestimated due to the sensitivity of these issues culturally and religiously in Oman.
Conclusion
The risk factors for HBV infection in Oman include direct contact with infected individuals within a family and exposure to high-risk behaviors such as piercing and barber shaving. While further analytical epidemiological studies are needed to assess the proportion of hepatitis B attributable to different risk factors, implementing antenatal screening for pregnant women would reduce the vertical transmission of HBV. In addition, improving contact screening would reduce horizontal transmission of the virus and reduce morbidity and mortality associated with the virus. Future work is required to confirm the association with behavioral risk factors and whether this association is causal, particularly piercing and shaving at barbershops. If confirmed, relatively simple and effective interventions could be developed to reduce the risk of horizontal transmission related to these activities.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018 Jun;3(6):383-403.
- 2. McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology 2009 May;49(5)(Suppl):S45-S55.
- 3. World Health Organization. Global health estimates: deaths by cause, age, sex, by country and by region, 2000–2015. 2015 [cited 2018 April 20]. Available from: http://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html.
- 4. Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, Kowdley KV. Global epidemiology of hepatitis B virus. J Clin Gastroenterol 2004 Nov-Dec;38(10)(Suppl 3):S158-S168.
- 5. Al-Naamani K, Al-Maqbali A, Al-Sinani S. Characteristics of hepatitis B infection in a sample of Omani patients. Sultan Qaboos Univ Med J 2013 Aug;13(3):380-385.
- 6. Shiffman ML. Management of acute hepatitis B. Clin Liver Dis 2010 Feb;14(1):169-176.
- 7. Toukan A; The Middle East Regional Study Group. Strategy for the control of hepatitis B virus infection in the Middle East and North Africa. Vaccine 1990 Mar;8(Suppl):S117-S121.
- 8. World Health Organization. Hepatitis B. 2002 [cited 2014 January 29]. Available from: http://www.who.int/csr/disease/hepatitis/whocdscsrlyo20022/en/.
- 9. Ali SA, Donahue RM, Qureshi H, Vermund SH. Hepatitis B and hepatitis C in Pakistan: prevalence and risk factors. Int J Infect Dis 2009 Jan;13(1):9-19.
- 10. Al-Rabeei NA, Al-Thaifani AA, Dallak AM. Knowledge, attitudes and practices of barbers regarding hepatitis B and C viral infection in Sana’a city, Yemen. J Community Health 2012 Oct;37(5):935-939.
- 11. Eroglu C, Zivalioglu M, Esen S, Sunbul M, Leblebicioglu H. Detection of hepatitis B virus in used razor blades by PCR. Hepat Mon 2010;10(1):22-25.
- 12. Alswaidi FM, O’Brien SJ. Is there a need to include HIV, HBV and HCV viruses in the Saudi premarital screening program on the basis of their prevalence and transmission risk factors? J Epidemiol Community Health 2010 Nov;64(11):989-997.
- 13. Jahangirnezhad MM, Hajiani EM, Makvandi MP, Jalali FB. A study on risk factors of chronic hepatitis B carriers. Jundishapur J Microbiol 2011;4:267-272.
- 14. Ministry of Health. Oman viral hepatitis survey. Community health & disease surveillance newsletter. Muscat: Ministry of Health. 2005 [cited 2014]. Available from: www.moh.gov.om/en/reports/publications/Newsletter14-2.pdf.
- 15. Ministry of Health. Infection prevention & control manual 1st addition. 1994 [cited 2014]. Available from: http://www.moh.gov.om/en/mgl/Manual/Communicable%20Diseases%20Surveillance.htm#sec7.
- 16. Alavian SM, Tabatabaei SV, Ghadimi T, Beedrapour F, Kafi-Abad SA, Gharehbaghian A, et al. Seroprevalence of hepatitis B virus infection and its risk factors in the west of Iran: a population-based study. Int J Prev Med 2012 Nov;3(11):770-775.
- 17. Merat S, Rezvan H, Nouraie M, Abolghasemi H, Jamali R, Amini-Kafiabad S, et al. Seroprevalence and risk factors of hepatitis A virus infection in Iran: a population based study. Arch Iran Med 2010 Mar;13(2):99-104.
- 18. Abdo AA, Sanai FM, Al-Faleh FZ. Epidemiology of viral hepatitis in Saudi Arabia: are we off the hook? Saudi J Gastroenterol 2012 Nov-Dec;18(6):349-357.
- 19. Memish ZA, Knawy BA, El-Saed A. Incidence trends of viral hepatitis A, B, and C seropositivity over eight years of surveillance in Saudi Arabia. Int J Infect Dis 2010 Feb;14(2):e115-e120.
- 20. Robinson T, Bullen C, Humphries W, Hornell J, Moyes C. The New Zealand hepatitis B screening programme: screening coverage and prevalence of chronic hepatitis B infection. N Z Med J 2005 Mar;118(1211):U1345.
- 21. Yang BM, Paik SW, Hahn OS, Yi DH, Choi MS, Payne S. Economic evaluation of the societal costs of hepatitis B in South Korea. J Gastroenterol Hepatol 2001 Mar;16(3):301-308.
- 22. Janahi EM. Prevalence and risk factors of hepatitis B virus infection in Bahrain, 2000 through 2010. PLoS One 2014 Feb;9(2):e87599.
- 23. Al-Shamahy H. Prevalence of hepatitis B surface antigen and risk factors of HBV infection in a sample of healthy mothers and their infants in Sana’a, Yemen. Ann Saudi Med 2000 Sep-Nov;20(5-6):464-466.
- 24. Gasim GI, Murad IA, Adam I. Hepatitis B and C virus infections among pregnant women in Arab and African countries. J Infect Dev Ctries 2013 Aug;7(8):566-578.
- 25. Urganci N, Akyildiz BN, Kalyoncu D, Gulec SG. Familial clustering of HBV in families with children who are diagnosed as chronic hepatitis B or inactive carriers of HBV. J Child Health Care 2013 Jun;17(2):197-203.
- 26. Ben-Alaya-Bouafif N, Bahri O, Chlif S, Bettaieb J, Toumi A, Bel Haj HN, et al. Heterogeneity of hepatitis B transmission in Tunisia: risk factors for infection and chronic carriage before the introduction of a universal vaccine program. Vaccine 2010 Apr;28(19):3301-3307.
- 27. Zhang Y, Fang W, Fan L, Gao X, Guo Y, Huang W, et al. Hepatitis B surface antigen prevalence among 12,393 rural women of childbearing age in Hainan Province, China: a cross-sectional study. Virol J 2013 Jan;10(1):25.
- 28. Zhang HW, Yin JH, Li YT, Li CZ, Ren H, Gu CY, et al. Risk factors for acute hepatitis B and its progression to chronic hepatitis in Shanghai, China. Gut 2008 Dec;57(12):1713-1720.
- 29. Urbanus AT, van den Hoek A, Boonstra A, van Houdt R, de Bruijn LJ, Heijman T, et al. People with multiple tattoos and/or piercings are not at increased risk for HBV or HCV in The Netherlands. PLoS One 2011;6(9):e24736.
- 30. Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol 2005 Dec;34(6):1329-1339.
- 31. Goldstein ST, Alter MJ, Williams IT, Moyer LA, Judson FN, Mottram K, et al. Incidence and risk factors for acute hepatitis B in the United States, 1982-1998: implications for vaccination programs. J Infect Dis 2002 Mar;185(6):713-719.
- 32. Al Awaidy S, Abu-Elyazeed R, Al Hosani H, Al Mulla A, Al Busaiedy S, Al Amiry A, et al. Sero-epidemiology of hepatitis B infection in pregnant women in Oman, Qatar and the United Arab Emirates. J Infect 2006 Mar;52(3):202-206.
- 33. Troung A, Walker S. Management of hepatitis B in pregnancy. The Royal Australian and New Zealand College of Obstetricians and Gynaecologists; 2013.
- 34. Hu Y, Zhang S, Luo C, Liu Q, Zhou YH. Gaps in the prevention of perinatal transmission of hepatitis B virus between recommendations and routine practices in a highly endemic region: a provincial population-based study in China. BMC Infect Dis 2012 Sep;12(1):1-7.
- 35. Yu M, Jiang Q, Ji Y, Jiang H, Wu K, Ju L, et al. The efficacy and safety of antiviral therapy with lamivudine to stop the vertical transmission of hepatitis B virus. Eur J Clin Microbiol Infect Dis 2012 Sep;31(9):2211-2218.
- 36. Alavian SM, Fallahian F, Lankarani KB. The changing epidemiology of viral hepatitis B in Iran. J Gastrointestin Liver Dis 2007 Dec;16(4):403-406.
- 37. Ministry of Health, Sultanate of Oman. Annual health report. 2012 [cited 30 January 2014]. Available from: http://www.moh.gov.om/en/stat/2012/Chapters/CH08Y12.pdf.