Colorectal cancer (CRC) is the most common cancer in Malaysia, and most prevalent cancer among men (16.4%), and the second most common cancer among women (10.7%).1 In Kelantan, the incidence of CRC shows an increasing trend from 1987 to 2007.2
CRC commonly occurs in the older age group with a mean age of 61.6 years.3 Globally, approximately 5% of CRC were young-onset.4 The incidence of CRC in this young population was reported to range from 0.4% to 35.6% relative to the total number of patients with CRC.5 In Malaysia, the incidence increased from 5% to 7% from 2008 to 2013.3
The microsatellite instability (MSI) pathway contributes to 15% of sporadic young CRC and more than 95% of hereditary nonpolyposis CRC (HNPCC) or Lynch syndrome. MSI occurs due to the defect in the DNA mismatch repair (MMR) system that eventually leads to an increased mutation rate in colorectal mucosa cells.
MMR proteins are nuclear enzymes important in repairing the base–base mismatch that occurs during DNA replication. It helps initiate the process of removing abnormal DNA by binding to abnormal DNA and forming complexes. Loss of MMR protein will result in accumulation of DNA replication error known as MSI.6
MSI can be categorized as MSI-high (MSI-H), MSI-low (MSI-L), or microsatellite stable (MSS).7 MSI-H is defined as the presence of a tumor with two or more mutated microsatellite sequences. MSI-L occurs when a tumor has only one mutated microsatellite sequence, and MSS describes the absence of mutation within the microsatellite panel.
Among all colorectal tumors with MSI, a large proportion is via the acquired methylation of MLH1 alone.7,8 Tumors with hypermethylation of MLH1 and BRAF mutation almost always represent sporadic CRC, which is not associated with HNPCC (Lynch syndrome).8 The characteristic features of sporadic CRC with MSI include the absence of family history with the loss of MLH1 and/or PMS2 expression, and the presence of BRAF (V600E) mutation.9
Sporadic young-onset CRCs are primarily due to epigenetic silencing of the MLH1 gene promoter. Sporadic MSI-H CRC harbors the V600E mutation of the BRAF oncogene. BRAF oncogene is a member of the RAF family involved in the mediation of cellular response to the growth signal through the RAS–RAF–MAP kinase pathway.10 BRAF V600E mutation is typically observed in sporadic tumors and not in HNPCC.11,12
The use of BRAF V600E mutation status has been demonstrated to differentiate MLH1-deficient sporadic CRC from HNPCC due to germline MLH1 mutation.11 BRAF V600E mutation status is also an adverse prognostic biomarker in patients with stage IV CRC, particularly those with MMR-proficient tumors.13
We aimed to determine the MMR protein expression (MLH1, MSH2, MSH6, PMS2, and BRAF V600E mutation) in the early-onset CRC in the Kelantan population. With the absence of MLH1 and/or PMS2 staining, positive BRAF V600E expression, and negative family history of CRC, we assumed that the patient was likely to have acquired MLH1 gene methylation, indicating the sporadic cause of CRC. We also aimed to create awareness for the early detection of CRC by recognizing the characteristic features of sporadic young CRC in the Kelantan population.
Methods
We conducted a cross-sectional study in the Hospital Universiti Sains Malaysia (USM) and Hospital Raja Perempuan Zainab II from 1 January 2006 to 31 December 2017. The study was approved by the human ethical committee of the School of Medical Sciences, USM Medical Research and Ethics Committee (MREC), Ministry of Health in Malaysia.
We investigated 31 patients with sporadic young-onset CRC. The definition of young-onset CRC varies in the literature. Most articles defined young as aged < 40 years.5,14,15 Therefore, 40 years old was used as a cut-off point to define young-onset in our study. Patients’ clinicopathological features were retrieved from the medical record unit.
We aimed to analyze the characteristic features of those who had no known risk factor at the time of diagnosis (such as HNPCC), as reported in the previous literature.8 HNPCC is an autosomal dominant genetic disorder characterized by a young-onset CRC that fulfills the Amsterdam criteria. These criteria include three patients with CRC in a family in which one individual was a first-degree relative of the other two. CRC occurred in at least two generations, and one affected family member was < 50 years.16 CRC in HNPCC syndrome is determined by a germline mutation in one of MMR components.
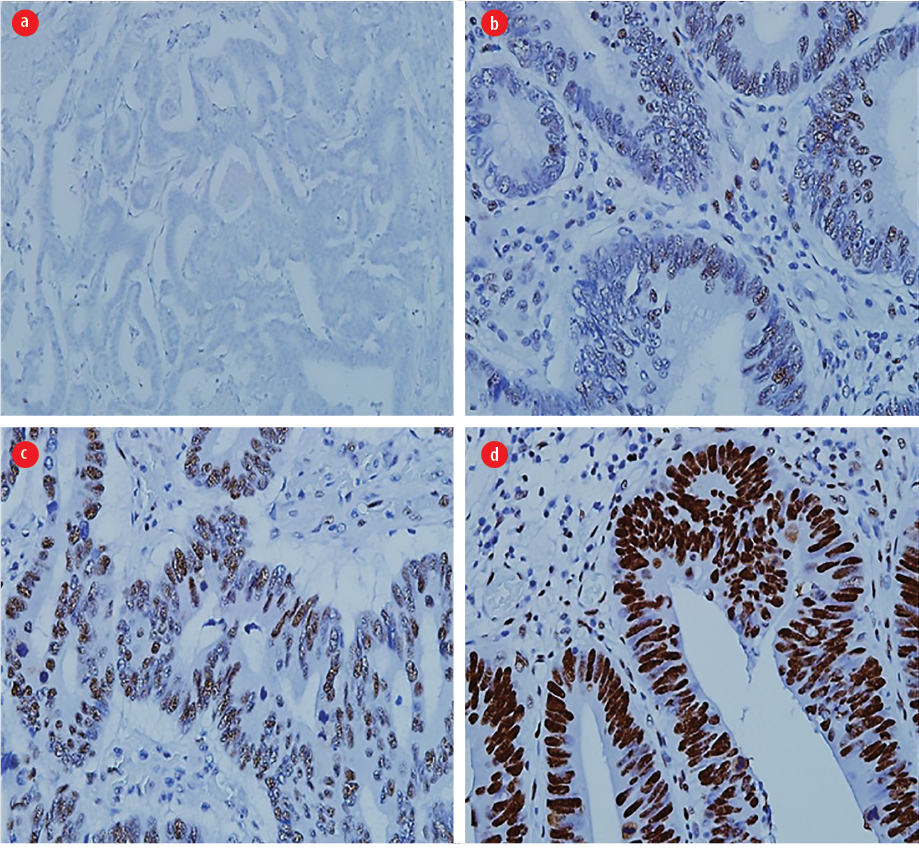
Figure 1: Nuclear intensity scoring for the mismatch repair protein expression (MLH1, MSH2, MSH6, and PMS2).(a) Score 0 (magnification = 100 ×), (b) score 1 (magnification = 400 ×), (c) score 2 (magnification = 400 ×), and (d) score 3 (magnification = 400 ×). The nuclear intensity was compared with the internal positive control (lymphocytes). A score of 3 indicates intensity as strong as the internal positive control.
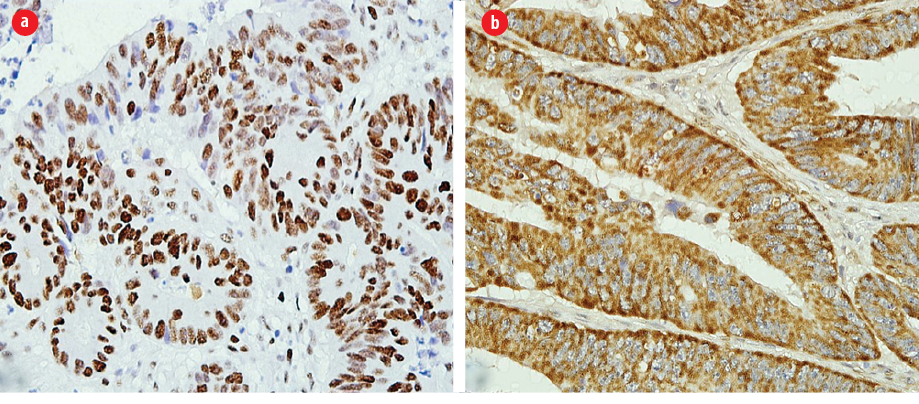
Figure 2: Representative staining results for the mismatch repair (MMR) protein expression and BRAF mutation through immunohistochemistry. (a) Colorectal sections using an antibody towards the MMR protein showing positive nuclear staining (magnification = 400 ×). (b) Colorectal cancer sections using an anti-BRAF V600E antibody, showing positive cytoplasmic staining (magnification = 400 ×).
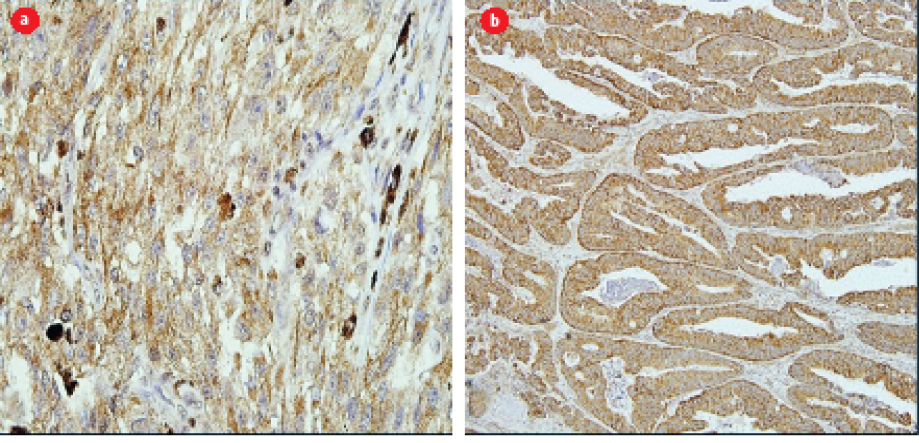
Figure 3: Positive control for the anti-BRAF V600E antibody. (a) Melanoma tissue used as positive control showing positive cytoplasmic staining for anti-BRAF V600E antibody (magnification = 400 ×). (b) Positive cytoplasmic staining was observed in the colorectal cancer tissue (magnification = 400 ×). Twenty-six out of 31 patients show positivity towards the anti-BRAF V600E antibody.
Using the above criteria, patients who fulfilled such criteria for HNPCC were excluded.16 In 1999, a revised criteria was published, including non-colonic tumors.17
A performa form was used to standardize the data collection and immunohistochemical scoring. Blocks of formalin-fixed paraffin-embedded tissue comprising an area of CRC tissue with adjacent normal colorectal mucosa were selected from the archive.
The selected tissue blocks were sectioned into 4 μm and placed on poly-L-lysine slides. After deparaffinization, antigen retrieval was carried using a heat-induced method (pH 9). Polyclonal primary antibodies were applied and incubated for 60 minutes at room temperature. Monoclonal rabbit anti-human MLH1, MSH2, MSH6, and PMS2 (Dako, Denmark), and monoclonal rabbit anti-human (anti-BRAF (mutated V600E) antibody [RM8] (ab200535) (Abcam, UK) were used.
For secondary antibody and detection system, horseradish peroxidase (HRP) polymer solution and 3,3-diaminobenzidine from DAKO were applied to the sections and incubated for one minute at room temperature. Slides were analyzed under a microscope (Olympus CX31) at 400 × magnification using semiquantitative scoring for MMR and BRAF antibodies.
Scoring for MLH1, MSH2, MSH6, and PMS2 immunohistochemical markers was based on the method adopted from Felsberg et al18 Their nuclear immunoreactivity was evaluated semiquantitatively and categorized based on score 0 (no cell positivity), score 1 (1–10% positive tumor cells), score 2 (11–50% positive tumor cells), and score 4 (> 80% positive tumor cells). The intensity of nuclear staining of malignant epithelial cells was measured on a scale of 0 to 3 and compared to the intensity of the positive control cells. A score 0 indicated no reactivity, and 3 denoted an intensity of tumor cells equivalent to the positive control. Both the nuclear score and the percentage of tumor positivity were multiplied to determine the status of the MMR protein as suggested by Barrow et al,19 A total score of ≥ 4 is positive while < 4 is negative, as shown in Figures 1 and 2.
The staining pattern for anti-BRAF V600E antibody is cytoplasmic staining. The tumor was scored positive when unequivocal cytoplasmic staining was seen. For any nuclear staining, weak cytoplasmic staining of isolated tumor cells or no staining was scored as negative. BRAF V600E mutation staining was scored as positive when > 80% of tumor cells showed immunopositivity [Figures 2b and 3].11
All the data obtained were analyzed using SPSS Statistics (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.). The association between clinicopathological characteristics with loss of MMR protein expression and positive BRAF V600E expression were analyzed using Fisher’s exact and Kruskal–Wallis tests. The level of significance was set at 0.050.
Results
A total of 31 cases were included, with the mean age of 31.5 years (range = 17–39 years). The frequency of sporadic young-onset CRC increased from 2006 to 2017 with the peak number of cases diagnosed in 2017 (six cases).
The sociodemographic profiles of cases were presented in Table 1; 61.3% were female, while 38.7% were male. The majority of CRC patients complained of abdominal pain (41.9%), followed by rectal bleeding (6.5%), constipation (6.5%), dark-colored stool (3.2%), and diarrhea (3.2%). Twelve (38.7%) cases had unknown presenting symptoms. Twenty-two (71.0%) cases were located on the right side of the colon, while nine (29.0%) cases were on the left side.
For histopathological subtype, 83.9% were moderately differentiated adenocarcinoma, 9.7% were mucinous adenocarcinoma, 3.2% were poorly differentiated adenocarcinoma, and 3.2% were signet ring cell carcinoma [Table 1]. Majority (54.8%) of cases presented with stage IV, 29.0% with stage III, and 16.1% with stage II.
Table 1: Sociodemographic characteristics among patients with young-onset colorectal cancer (n = 31).
|
Age, years |
31.5 ± 5.8* |
|
Race |
|
|
Malay |
31 (100.0) |
|
Gender |
|
|
Male |
12 (38.7) |
|
Female |
19 (61.3) |
|
Symptoms |
|
|
Abdominal pain |
13 (41.9) |
|
Rectal bleeding/bloody stool |
2 (6.5) |
|
Dark-colored stool |
1 (3.2) |
|
Constipation |
2 (6.5) |
|
Diarrhea |
1 (3.2) |
|
Unknown |
12 (38.7) |
|
Location |
|
|
Right side |
22 (71.0) |
|
Left side |
9 (29.0) |
|
Histology |
|
|
Moderately differentiated adenocarcinoma |
26 (83.9) |
|
Mucinous adenocarcinoma |
3 (9.7) |
|
Signet ring cell carcinoma |
1 (3.2) |
|
Poorly differentiated adenocarcinoma |
1 (3.2) |
|
Stage |
|
|
II |
5 (16.1) |
|
III |
9 (29.0) |
|
IV |
17 (54.8) |
|
MMR protein |
|
|
MSS |
20 (64.5) |
|
MSI-L |
2 (6.5) |
|
MSI-H |
9 (29.0) |
|
BRAF V600E |
|
|
Positive |
26 (83.9) |
|
Negative |
3 (9.7) |
*mean ± standard deviation.
MMR: mismatch repair; MSS: microsatellite stable; MSI-L: low microsatellite instability; MSI-H: high microsatellite instability.
Table 2: Association between clinic-pathological characteristics with types of mismatch repair (MMR) protein expression and BRAF mutation status (n = 31).
|
Age |
32.5 (8.0) |
30.0 (0.0) |
34.0 (10.0) |
0.052a |
33.5 (8.0) |
25.0 (0.0) |
32.0 (0.0) |
0.373a |
|
Gender |
|
|
|
|
|
|
|
|
|
Male |
8 (66.7) |
1 (8.3) |
3 (25.0) |
> 0.950b |
9 (75.0) |
2 (16.7) |
1 (8.3) |
0.610b |
|
Female |
12 (63.2) |
1 (5.3) |
6 (31.6) |
|
17 (89.5) |
1 (5.3) |
1 (5.3) |
|
|
Abdominal pain |
|
|
|
|
|
|
|
|
|
Yes |
8 (61.5) |
2 (15.4) |
3 (23.1) |
0.817b |
10 (76.9) |
2 (15.4) |
1 (7.7) |
0.927b |
|
No |
4 (66.7) |
0 (0.0) |
2 (33.3) |
|
6 (100) |
0 (0.0) |
0 (0.0) |
|
|
Unknown |
8 (66.7) |
0 (0.0) |
4 (33.3) |
|
10 (83.3) |
1 (8.3) |
1 (8.3) |
|
|
Abdominal mass |
|
|
|
|
|
|
|
|
|
No |
12 (63.2) |
2 (10.5) |
|
0.712b |
16 (84.2) |
2 (10.5) |
1 (5.3) |
> 0.950b |
|
Unknown |
8 (66.7) |
0 (0.0) |
4 (33.3) |
|
10 (83.3) |
1 (8.3) |
1 (8.3) |
|
|
Rectal bleeding |
|
|
|
|
|
|
|
|
|
Yes |
2 (100) |
0 (0.0) |
0 (0.0) |
0.763b |
2 (100) |
0 (0.0) |
0 (0.0) |
> 0.950b |
|
No |
10 (58.8) |
2 (11.8) |
5 (29.4) |
|
14 (82.4) |
2 (11.8) |
1 (5.9) |
|
|
Unknown |
8 (66.7) |
|
4 (33.3) |
|
10 (83.3) |
1 (8.3) |
1 (8.3) |
|
|
Constipation |
|
|
|
|
|
|
|
|
|
Yes |
1 (50.0) |
0 (0.0) |
1 (50.0) |
0.720b |
2 (100.0) |
0 (0.0) |
0 (0.0) |
> 0.950b |
|
No |
11 (64.7) |
2 (11.8) |
4 (23.5) |
|
14 (82.4) |
2 (11.8) |
1 (5.9) |
|
|
Unknown |
8 (66.7) |
0 (0.0) |
4 (33.3) |
|
10 (83.3) |
1 (8.3) |
1 (8.3) |
|
|
Diarrhea |
|
|
|
|
|
|
|
|
|
Yes |
1 (100) |
0 (0.0) |
0 (0.0) |
0.811b |
1 (100) |
0 (0.0) |
0 (0.0) |
> 0.950b |
|
No |
11 (61.1) |
2 (11.1) |
5 (27.8) |
|
15 (83.3) |
2 (11.1) |
1 (5.6) |
|
|
Unknown |
8 (66.7) |
0 (0.0) |
4 (33.3) |
|
10 (83.3) |
1 (8.3) |
1 (8.3) |
|
|
Nausea vomiting |
|
|
|
|
|
|
|
> 0.950b |
|
No |
12 (63.2) |
2 (10.5) |
5 (26.3) |
0.712b |
16 (84.2) |
2 (10.5) |
1 (5.3) |
|
|
Unknown |
8 (66.7) |
0 (0.0) |
4 (33.3) |
|
10 (83.3) |
1 (8.3) |
1 (8.3) |
|
|
Fatigue |
|
|
|
|
|
|
|
|
|
No |
12 (63.2) |
2 (10.5) |
5 (26.3) |
0.712b |
16 (84.2) |
2 (10.5) |
1 (5.3) |
> 0.950b |
|
Unknown |
8 (66.7) |
0 (0.0) |
4 (33.3) |
|
10 (83.3) |
1 (8.3) |
1 (8.3) |
|
|
Weight loss |
|
|
|
|
|
|
|
|
|
No |
12 (63.2) |
2 (10.5) |
5 (26.3) |
0.712b |
16 (84.2) |
2 (10.5) |
1 (5.3) |
> 0.950b |
|
Unknown |
8 (66.7) |
0 (0.0) |
4 (33.3) |
|
10 (83.3) |
1 (8.3) |
1 (8.3) |
|
|
Loss of appetite |
|
|
|
|
|
|
|
|
|
No |
12 (63.2) |
2 (10.5) |
5 (26.3) |
0.712b |
16 (84.2) |
2 (10.5) |
1 (5.3) |
> 0.950b |
|
Unknown |
8 (66.7) |
0 (0.0) |
4 (33.3) |
|
10 (83.3) |
1 (8.3) |
1 (8.3) |
|
|
Dark stool |
|
|
|
|
|
|
|
|
|
Yes |
0 (0.0) |
0 (0.0) |
1 (100) |
0.370b |
1 (100) |
0 (0.0) |
0 (0.0) |
> 0.950b |
|
No |
13 (68.4) |
2 (10.5) |
4 (21.1) |
|
16 (84.2) |
2 (10.5) |
1 (5.3) |
|
|
Unknown |
7 (63.6) |
0 (0.0) |
4 (36.4) |
|
9 (81.8) |
1 (9.1) |
1 (9.1) |
|
|
Location |
|
|
|
|
|
|
|
|
|
Right side |
13 (59.1) |
2 (9.1) |
7 (31.8) |
0.839b |
17 (77.3) |
3 (13.6) |
2 (9.1) |
0.572b |
|
Left side |
7 (77.8) |
0 (0.0) |
2 (22.2) |
|
9 (100) |
0 (0.0) |
0 (0.0) |
|
|
Histology |
|
|
|
|
|
|
|
|
|
Moderately differentiated adenocarcinoma |
18 (69.2) |
1 (3.8) |
7 (26.9) |
0.183b |
24 (92.3) |
1 (3.8) |
1 (3.8) |
0.015b |
|
Mucinous adenocarcinoma |
1 (33.3) |
1 (33.3) |
1 (33.3) |
|
2 (66.7) |
1 (33.3) |
0 (0.0) |
|
|
Signet ring cell carcinoma |
0 (0.0) |
0 (0.0) |
1 (100) |
|
0 (0.0) |
1 (100) |
0 (0.0) |
|
|
Poorly differentiated adenocarcinoma |
1 (100) |
0 (0.0) |
0 (0.0) |
|
0 (0.0) |
0 (0.0) |
1 (100) |
|
|
Stage |
|
|
|
|
|
|
|
|
|
II |
3 (60.0) |
0 (0.0) |
2 (40.0) |
0.669b |
5 (100) |
0 (0.0) |
0 (0.0) |
0.914b |
|
III |
7 (77.8) |
1 (11.1) |
1 (11.1) |
|
8 (88.9) |
1 (11.1) |
0 (0.0) |
|
MSS: microsatellite stable; MSI-L: low microsatellite instability; MSI-H: high microsatellite instability.
.aFisher’s exact test was applied.; bKruskal–Wallis test was applied; p-value is significant (< 0.050).
From 31 cases, 20 (64.5%) cases expressed all four panels of MMR proteins (MSS), nine (29.0%) patients showed loss of more than one MMR protein expression (MSI-H), and two (6.5%) cases loss of one MMR protein expression (MSI-L). BRAF V600E positivity was seen in 26 (83.9%) cases while three (9.7%) cases were negative for BRAF V600E expression and two (6.5%) cases were equivocal [Table 1].
The distribution of cases based on the groups for MMR protein (MSS, MSI-H, MSI-L) and BRAF V600E expression are shown in Table 1. There were 64.5% (n = 20) of MSS and 83.9% (n = 26) of positive BRAF expression for V600E mutation discovered in the present study.
There were no significant associations between gender, symptoms, anatomic location, histology subtypes, and cancer stage with MMR protein expression [Table 2]. The majority of cases were moderately differentiated adenocarcinoma, and 92.3% were positive for BRAF mutation. Only the histology subtype showed a significant association with positive expression of BRAF V600E (p = 0.015).
Discussion
Molecular sub-classification of young CRC into sporadic and hereditary is important for the treatment, prognosis, and management of patients in the young age group. Molecular characterization is time-consuming, expensive, and requires the expert technical staff that have been trained in molecular diagnostic and specialize infrastructures associated with genetic testing.
The sub-classification of young-onset CRC into sporadic and Lynch syndrome is crucial. Early detection of hereditary nonpolyposis CRC can result in early screening of the affected family members. MMR proteins (MSH2, PMS2, PSH6, and MLH1) and BRAF are parameters included in the sub-classification of young-onset CRC. Currently, immunohistochemistry of MMR proteins (MLH1, PMS2, MSH2, and MSH6) and BRAF V600E has been developed to screen this young age group of CRC that demonstrates similar specificity and sensitivity with the polymerase chain reaction-based approach.20
MMR protein expression can be grouped into MSS, MSI-H, and MSI-L.7 In this study, 64.5% of sporadic young-onset CRC were MSS, defined as the presence of immunohistochemistry expression in all microsatellite panels used (MLH1, MSH2, MSH6, and PMS2). The MSI that includes both MSI-H and MSI-L was evident in 11 (35.5%) patients. A previous study showed a similar pattern of MMR protein expression in sporadic young-onset CRC. From their study, 256 of 310 patients were MSS (82.6%).21
Cytoplasmic staining was assessed in anti-BRAF V600E antibody. Positive staining was observed in 26 (83.9%) patients, whereas three (9.7%) patients were negative for BRAF V600E mutation immunohistochemistry expression. Two out of 31 patients showed an equivocal result. This outcome was due to positive BRAF V600E antibody at the normal surface of the colonic epithelium and tumor tissue. A study by Day et al,22 reported similar findings in the adjacent normal colonic tissue. Repeated staining were performed for these two patients with a shorter incubation time for the primary antibody yielding similar results. Only the intensity of the cytoplasmic staining was slightly reduced with a shorter incubation period.
Focal positive staining was also observed in these cases, contributing to interpretative difficulties. This was initially thought to be caused by unequal distribution of the antibody during the staining procedure. However, similar results were observed after performing repeated staining. Therefore, the confirmation of BRAF status should be verified using a more sensitive molecular method.
Present data analysis showed a significant association between histology subtypes and BRAF mutation (p = 0.015). The majority of patients had moderately differentiated adenocarcinoma, and 92.3% were positive for BRAF V600E expression. A previous study reported that the immunohistochemistry characteristic of sporadic CRC is loss of MLH1 and/or PMS2 with BRAF mutation.9 In this study, only four of 31 (12.9%) patients presented with this characteristic feature. PMS2 negative with positive BRAF protein mutation expression was observed in only one (3.2%) patient. From these patients (the absence of MLH1 and/or PMS2 staining with positive BRAF V600E expression and negative family history), the authors assumed that the patient is likely to have experienced methylation of the MLH1 gene promoter that acts as a sporadic cause for young CRC that needs further molecular study.
The frequency of BRAF V600E positive expression observed in patients with MSS was 17/20 (85.0%) and MSI tumors 9/11 (81.8%), indicating a big difference compared to that of a previous study reporting only 4% and 39% of patients, respectively.23 Further genetic testing of the MLH1 gene and/or BRAF mutation is recommended for confirmation.7 However, the positivity of BRAF V600E in these patients is in favor of sporadic young-onset CRC.
BRAF V600E immunohistochemistry expression is found in approximately two-thirds of CRC with loss of MLH1. Its occurrence with MSI due to Lynch syndrome is not yet reported. Genetic testing for suspected Lynch syndrome is offered to those who had MLH1 expression loss without BRAF mutation. BRAF V600E mutation in the MSS-stable group is a different entity that emerges as a poor prognosis.
Identifying MSI-H, MSI-L, or BRAF mutation in young-onset CRC is important for treatment selection and prognostic value. Without adjuvant chemotherapy, patients with MSI-H generally have better survival than those with MSS or MSI-L.24 However, patients with MSI-H may not benefit from fluorouracil-based adjuvant chemotherapy than those with MSS and MSI-L who showed improved overall survival.24 Furthermore, evidence shows that BRAF mutation is associated with a poorer prognosis.25
The present study had limitations in the sample size, as we had difficulties extracting CRC data due to the retrospective nature of this study. Some clinical notes were not available, and medical records were inactive due to non-compliance or defaulted follow-up and death. Another limitation is due to budget constraints; thus, we were unable to perform germline mutation analysis.
The authors would like to expand the sample size of the young-onset CRC by collaborating with other institutions in other states. In addition, molecular and genetic testing for these patients should be explored to understand the genetic component of young-onset CRC better.
Conclusion
A majority of patients with young-onset CRC present with abdominal pain at an advanced stage. Most tumors are moderately differentiated adenocarcinoma and located at the right side of the colon. They are predominantly MSS, in which they expressed all MMR proteins panel with a majority being positive for BRAF V600E expression. A significant association between histological findings and BRAF V600E positive expression was identified via the immunohistochemistry method. This knowledge and understanding will allow us to develop a clinicopathological profile for young-onset CRC, thereby providing the community with a better insight into the disease. Consequently, early detection and intervention can be implemented. This finding should shed light on further research on this disease.
Disclosure
The authors declared no conflicts of interest. This research was supported by a short-term grant from Universiti Sains Malaysia (304/PPSP/61313207).
Acknowledgements
The researchers would like to thank Universiti Sains Malaysia for funding with a short-term grant.
references
- 1. Azizah AM, Norsaleha IT, Noor Hashimah A, Asmah ZA, Mastulu W. Malaysian national cancer registry report 2007-2011. National Cancer Institute; 2016.
- 2. Othman NH, Nor ZM, Biswal BM. Is Kelantan joining the global cancer epidemic?–experience from hospital Universiti Sains Malaysia; 1987-2007. Asian Pac J Cancer Prev 2008 Jul-Sep;9(3):473-478.
- 3. Hassan MR, Khazim WK, Mustapha NR, Othman Z. National cancer patient registry-colorectal cancer in Malaysia. Ann Oncol 2013 Jun;24:iv97.
- 4. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015 Mar;136(5):E359-E386.
- 5. O’Connell JB, Maggard MA, Livingston EH, Yo CK. Colorectal cancer in the young. Am J Surg 2004 Mar;187(3):343-348.
- 6. Kheirelseid EA, Miller N, Chang KH, Curran C, Hennessey E, Sheehan M, et al. Mismatch repair protein expression in colorectal cancer. J Gastrointest Oncol 2013 Dec;4(4):397-408.
- 7. Kurzawski G, Suchy J, Dębniak T, Kładny J, Lubiński J. Importance of microsatellite instability (MSI) in colorectal cancer: MSI as a diagnostic tool. Ann Oncol 2004;15(Suppl 4):iv283-iv284.
- 8. Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010 Jun;138(6):2073-2087.
- 9. Batur S, Vuralli Bakkaloglu D, Kepil N, Erdamar S. Microsatellite instability and BRAF mutation in colorectal cancer: clinicopathological characteristics and effects on survival. Bosn J Basic Med Sci 2016;16:254-260.
- 10. Parsons MT, Buchanan DD, Thompson B, Young JP, Spurdle AB. Correlation of tumour BRAF mutations and MLH1 methylation with germline mismatch repair (MMR) gene mutation status: a literature review assessing utility of tumour features for MMR variant classification. J Med Genet 2012 Mar;49(3):151-157.
- 11. Capper D, Voigt A, Bozukova G, Ahadova A, Kickingereder P, von Deimling A, et al. BRAF V600E-specific immunohistochemistry for the exclusion of Lynch syndrome in MSI-H colorectal cancer. Int J Cancer 2013 Oct;133(7):1624-1630.
- 12. Thiel A, Heinonen M, Kantonen J, Gylling A, Lahtinen L, Korhonen M, et al. BRAF mutation in sporadic colorectal cancer and Lynch syndrome. Virchows Arch 2013 Nov;463(5):613-621.
- 13. Toon CW, Chou A, DeSilva K, Chan J, Patterson J, Clarkson A, et al. BRAFV600E immunohistochemistry in conjunction with mismatch repair status predicts survival in patients with colorectal cancer. Mod Pathol 2014 May;27(5):644-650.
- 14. Chan KK, Dassanayake B, Deen R, Wickramarachchi RE, Kumarage SK, Samita S, et al. Young patients with colorectal cancer have poor survival in the first twenty months after operation and predictable survival in the medium and long-term: analysis of survival and prognostic markers. World J Surg Oncol 2010 Sep;8(1):82.
- 15. Zbuk K, Sidebotham EL, Bleyer A, La Quaglia MP. Colorectal cancer in young adults. Semin Oncol 2009 Oct;36(5):439-450.
- 16. Vasen HF, Mecklin JP, Khan PM, Lynch HT. The international collaborative group on hereditary nonpolyposis colorectal cancer (ICG-HNPCC). Dis Colon Rectum 1991 May;34(5):424-425.
- 17. Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the international collaborative group on HNPCC. Gastroenterology 1999 Jun;116(6):1453-1456.
- 18. Felsberg J, Thon N, Eigenbrod S, Hentschel B, Sabel MC, Westphal M, et al; German Glioma Network. Promoter methylation and expression of MGMT and the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2 in paired primary and recurrent glioblastomas. Int J Cancer 2011 Aug;129(3):659-670.
- 19. Barrow E, Jagger E, Brierley J, Wallace A, Evans G, Hill J, et al. Semiquantitative assessment of immunohistochemistry for mismatch repair proteins in Lynch syndrome. Histopathology 2010 Feb;56(3):331-344.
- 20. Toon CW, Walsh MD, Chou A, Capper D, Clarkson A, Sioson L, et al. BRAFV600E immunohistochemistry facilitates universal screening of colorectal cancers for Lynch syndrome. Am J Surg Pathol 2013 Oct;37(10):1592-1602.
- 21. Ward R, Meagher A, Tomlinson I, O’Connor T, Norrie M, Wu R, et al. Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut 2001 Jun;48(6):821-829.
- 22. Day F, Muranyi A, Singh S, Shanmugam K, Williams D, Byrne D, et al. A mutant BRAF V600E-specific immunohistochemical assay: correlation with molecular mutation status and clinical outcome in colorectal cancer. Target Oncol 2015 Mar;10(1):99-109.
- 23. Li WQ, Kawakami K, Ruszkiewicz A, Bennett G, Moore J, Iacopetta B. BRAF mutations are associated with distinctive clinical, pathological and molecular features of colorectal cancer independently of microsatellite instability status. Mol Cancer 2006 Jan;5(1):2.
- 24. Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003 Jul;349(3):247-257.
- 25. Xu Q, Xu AT, Zhu MM, Tong JL, Xu XT, Ran ZH. Predictive and prognostic roles of BRAF mutation in patients with metastatic colorectal cancer treated with anti-epidermal growth factor receptor monoclonal antibodies: a meta-analysis. J Dig Dis 2013 Aug;14(8):409-416.