|
Abstract
Objectives: Aminoglycosides are highly effective against bacteria but have serious side-effects including ototoxicity and nephrotoxicity. One of the theories in aminoglycosides ototoxicity is that Iron-aminoglycoside complex causes ototoxicity by creating free radicals. Based on this theory, the relationship between serum iron level and amikacin ototoxicity was studied to determine whether more iron results in more ototoxcity.
Methods: This prospective cohort study was conducted from August 2005 to October 2008. Patients with amikacin prescription and different serum-ferritin levels were examined. Burned patients with amikacin prescription were divided into Group1 (89 patients; serum-ferritin >150) and Group2 (92 patients, serum-ferritin <150). Their hearing thresholds and red-blood-cells indices were compared using t- and paired t-test.
Results: In comparing the two groups, thresholds of Group1 were higher than Group2 at all frequencies, and the difference was statistically significant (p<0.001). The maximum threshold shift in Group1 was greater than 20 dB and in Group2, it was less than 10 dB, at 8000Hz. Again, this result was statistically and clinically significant (p<0.001). Finally, the mean corpuscular volume (MCV)was higher in Group1 than Group2, and (p=0.001).
Conclusion: The results suggest that the level of iron is related to aminoglycoside ototoxicity. More iron can create more ototoxicity, and iron deficiency may inhibit aminoglycoside ototoxicity. An increase in MCV may be due to higher serum ferritin and an indication of more ototoxicity.
Keywords: Antibiotics; Audiology; Hearing loss.
Introduction
Aminoglycosides are one of the most effective antimicrobial agents against the majority of bacteria. Amikacin is classified as deoxystreptamine of aminoglycosides and has strong toxicity for cochlea.1 Aminoglycosides present serious side effects such as nephrotoxicity and ototoxicity. Ototoxicity has been reported in 10% to 80% of patients. Early screening by high-frequency audiometery (16 kHz) showed a detectable hearing loss in 33% of patients.2 The studies have shown that the formation of iron-aminoglycoside complex produces free radicals. These free radicals cause ototoxicity by damaging the outer hair cells of the inner ear.3 Iron chelators can protect cochlear and vestibular against the side effects of Aminoglycosides by inhibiting the formation of free radicals. Iron chelators do not affect the antibacterial efficacy of aminoglycosides.4,5 Free iron is toxic for cells and cells have a protective mechanism as binding iron (e.g., ferritin and hemosiderin). In a steady-state condition, serum ferritin level correlates with the body's total iron storage. Serum ferritin level is the most convenient and useful laboratory test for estimating the body's iron storage. The normal level of serum ferritin varies according to age and gender of individuals. Less than 150 ng/mL of serum ferritin is diagnosed as the absence of the body's iron storage.6 Aminoglycoside-iron complex formation increases by the available iron.7,8 In this study, the relationship between hearing loss (ototoxicity) by amikacin and serum iron level was investigated.
Methods
This prospective cohort study was conducted from August 2005 to October 2008. Patients due to receive amikacin (10 mg/kg/day) for wound infections in the burns ward were selected in the city of Hamadan by sequential sampling. The selected patients had no history of ear diseases or trauma, nor did they have ear surgery, abnormal ear examination, use ototoxic drugs and/or renal failure. The patient's creatinine levels were within normal range. CBC and serum ferritin lab tests were done for all patients before receiving amikacin. Patients were divided into two groups in terms of serum ferritin levels (serum ferritin >150 ng/ml as Group1 and serum ferritin <150 ng/mL as Group2) which were measured by Enzyme Linked Immunosorbent Assay (ELISA) laboraty method. All of the patients were assessed by audiologic tests as pure tone audiometry (PTA) and speech discrimination score (SDS) using the AC 40 (Interacostics, Assen-Denmark) audiometer by a professional audiologist. Audiologic assessments were done before receiving amikacin injections and 6 weeks after amikacin injections. In PTA, the patients' hearing threshold levels were assessed at frequencies of 250, 500, 1000, 2000, 4000, and 8000Hz. The results were analyzed by t-test and paired t-test in SPSS 16. A p-value of <0.05 was considered significant. This study was approved by the ethics committee at the Hamedan University of Medical Sciences.
Results
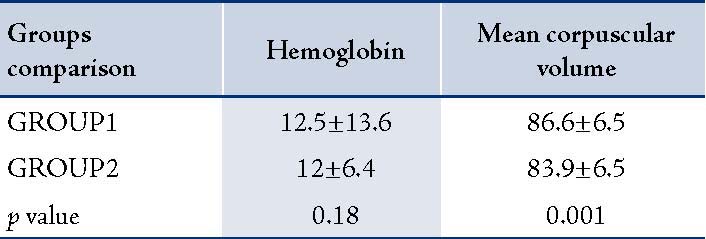
A total of 181 patients were studied. Group1 consisted of 89 cases (34 females and 55 males) with a mean age 27±12.8 years, while Group2 consisted of 92 cases (35 females and 57 males) with a mean age of 27.5±13.4 years. In Group1, the mean serum ferritin level was 317±102.8 ng/mL and in Group2, it was 83/4±53.4 ng/mL. Mean duration of receiving amikacin was 12±5.6 days for Group1 and 12.5±6.1 days for Group2. There was no statistically significant difference in age, sex and the duration of Amikacin injections between Group1 and Group2 (p=0.1). Pre-treatment hearing thresholds were approximately 10 dB in all frequencies in both groups but the difference between them was not statistically significant (p=0.2). Both groups had an increase in the mean thresholds in all frequencies after treatment with amikacin (p<0.001). However, there was no difference between the thresholds in both ears in pre and post treatment. The average threshold in both ears are presented in Figure 1. Group1 (serum ferritin >150 ng/mL) had greater increase in thresholds than Group2 (p<0.001). The maximum threshold shift was at 8000Hz (in Group1 the maximum threshold was greater than 20 dB and in Group2 it was less than 10 dB). Higher frequencies exhibited greater damage than lower frequencies in both groups. Thus, suggesting that the mean threshold shift is dependent on amikacin (Table 1). Hemoglobin (Hgb), hematocrit (Hct), the mean corpuscular hemoglobin (MCH), and the mean corpuscular hemoglobin concentration (MCHC) were within normal range in both groups and the difference between the two groups was not significant (p=0.18). MCV was greater in Group1 than Group2 and the difference was significant (p=0.001).(Table 2).
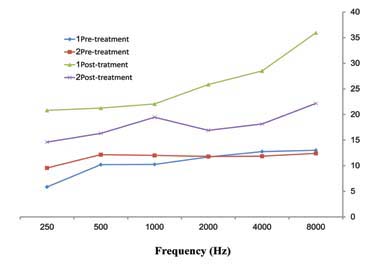
Figure 1: The comparison of mean values PTA (dB) frequencies (Hz) in pre and post-Amikacin injections by considering average of both ears in 181 burned patients in Hamedan.
Table 1: The comparison of mean values PTA frequencies in pre and post-Amikacin injections in 181 burned patients in Hamedan
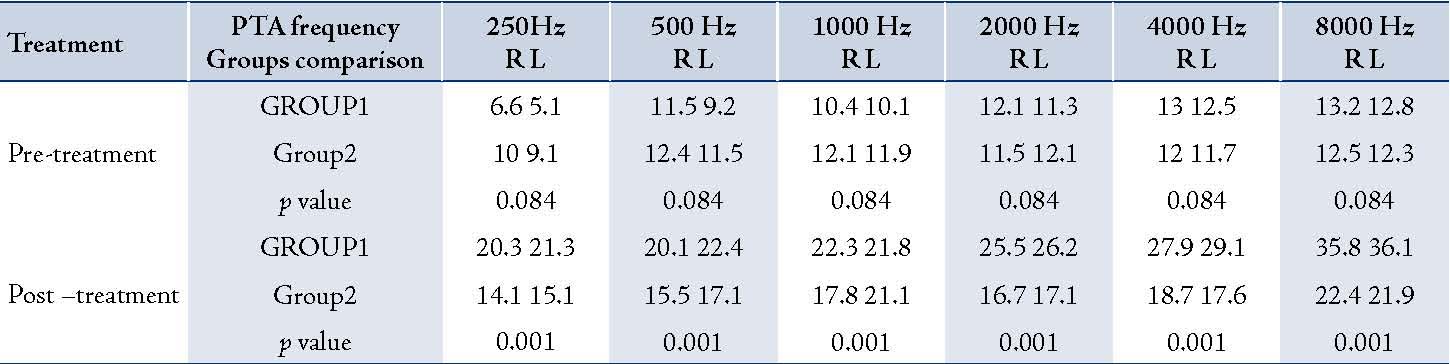
Table 2: The comparison of hemoglobin and mean corpuscular volume in two groups.
Discussion
Although aminoglycoside antibiotics are some of the most commonly used agents for treatment of gram-negative bacterial infections and tuberculosis, it has long been known that the major irreversible side effect of amino glycosides is ototoxicity. One of the theories in Aminoglycoside ototoxicity is that iron-aminoglycoside complex causes inner ear damage by generating free radicals.1 The damage in hearing are caused by the destruction of outer hair cells and neurons similar to cispalatin toxicity, noise induced hearing loss and perhaps the age-related hearing impairment.9 The formation of iron-aminoglycoside complex for the creation of free radical species hav been suggested.
Numerous studies have demonstrated that free radical scavengers and iron chelators tend to attenuate the ototoxic effects of aminoglycosides,10 but deferoxamine is thought to cause ototoxic effects by itself. Reports have shown that ototoxic actions of gentamicin may be mediated by iron chelation.11 Also it has been reported that iron supplementation, by increasing serum iron levels, exacerbates aminoglycoside ototoxicity in vivo.12
Conclusion
The study results were similar to those repoerted by Song, Mostafa and Conlon.3,4,11 In the current study, the MCV of red blood cells was higher in Group1 (p=0.001). The increase in MCV may have been as result of higher serum iron levels irrespective of the normal ferittin range.
This study confirms the role of iron in aminoglycoside ototoxicity even though serum amikacin levels were not measured. Thus, more iron can create more ototoxicity and iron deficiency may inhibit aminoglycoside-ototoxicity. The results suggest that if a patient has iron deficiency anemia, and uses aminoglycoside without any serious problem with their anemia; then it’s better to delay the prescription of iron supplement after treatment with aminoglycoside. However, if patients must be given iron, they should be followed-up for hearing loss and their aminoglycoside serum levels should be closely monitored. Also, we recommend that high-resolution audiometry and auditory brainstem response (ABR) for particular aminoglycoside ototoxicity be conducted.
Acknowledgements
The authors reported no conflict of interest and no funding was received in this work.
References
1. Crann SA, Schacht J. Activation of aminoglycoside antibiotics to cytotoxins. Audiol Neurootol 1996 Mar-Apr;1(2):80-85.
2. Priuska EM, Schacht J. Formation of free radicals by gentamycin and iron and evidence for an iron/gentamycin complex. Biochem Pharmacol 1995;50:1746-1752 .
3. Asplund MS, Lidian A, Linder B, Takumida M, Anniko M. Protective effect of edaravone against tobramycin-induced ototoxicity. Acta Otolaryngol 2009 Jan;129(1):8-13.
4. Song BB, Anderson DJ, Schacht J. Protection from gentamicin ototoxicity by iron chelators in guinea pig in vivo. J Pharmacol Exp Ther 1997 Jul;282(1):369-377.
5. Mostafa BE, Tawfik S, Hefnawi NG, Hassan MA, Ismail FA. The role of deferoxamine in the prevention of gentamicin ototoxicity: a histological and audiological study in guinea pigs. Acta Otolaryngol 2007 Mar;127(3):234-239.
6. Hillman RS. 2005. Hematology in clinical practice, 4 th ed. New York McGraw-Hill.
7. Clerici WJ, DiMartino DL, Prasad MR. Direct effects of reactive oxygen species on cochlear outer hair cell shape in vitro. Hear Res 1995 Apr;84(1-2):30-40.
8. Hirose K, Hockenbery DM, Rubel EW. Reactive oxygen species in chick hair cells after gentamicin exposure in vitro. Hear Res 1997 Feb;104(1-2):1-14.
9. Schacht J. 1993Biocheemical basis of ototoxcity. In:Rybak,L.P.,ed.Otolaryngologic clinics of North America, Vol. 26. Philadelphia: W.B. Saunders. 845-856.
10. Song BB, Schacht J. Variable efficacy of radical scavengers and iron chelators to attenuate gentamicin ototoxicity in guinea pig in vivo. Hear Res 1996 May;94(1-2):87-93.
11. Ryals B, Westbrook E, Schacht J. Morphological evidence of ototoxicity of the iron chelator deferoxamine. Hear Res 1997 Oct;112(1-2):44-48.
12. Conlon BJ, Smith DW. Supplemental iron exacerbates aminoglycoside ototoxicity in vivo. Hear Res 1998 Jan;115(1-2):1-5.
|