|
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a common endocrine metabolic disease which occurs in 5-10% of reproductive age women.1-3 PCOS is an inherited disease affecting women of childbearing age.4 The disorder causes multiple abnormal cysts and enlarged ovaries, so that they do not produce the normal number of eggs and do not ovulate normally. The disease is present at birth but is not cause symptomatic until a genetic component and clinical feature of this disorder change throughout the life span, starting from adolescence to postmenopausal age. Hence, no effort has been made to define the differences in the phenotype and clinical presentation according to age.5,6
The course of treatment for women with PCOS largely depends on the severity of an individual’s symptoms. Well-defined published data indicate a high risk for the development of Type 2 diabetes mellitus (T2DM) and Cardiovascular Disease (CVD) in women with PCOS. In view of the lack of protective effect of female sex on CVD risk in patients with diabetes, the associated risks of CVD are magnified in women with diabetes who have PCOS.7,8 Clearly, this situation means that PCOS is a general health disorder for young women, with potential for reversal of some of the associated risk with early diagnosis and treatment.9 Lifestyle modification with weight loss and exercise, avoidance of tobacco, correction of lipid abnormalities, and use of metformin may be of value. Metformin therapy does not only reduce hyperinsulinism and improve steroidogenic dysfunction, but also it is helpful in achieving better regularity of menses and fertility potential.10,11 Thiazolidinediones have also been shown to decrease androgen levels, improve ovulation, and reduce progression to overt T2DM in patients with PCOS and impaired glucose tolerance (IGT).12-14
Metformin (N, N-dimethylimidodicarbonimidic diamide) is now thought to be of therapeutic value directly and/or indirectly in the management of PCOS.15 It is the first-line drug of choice for the treatment of T2DM, particularly in overweight and obese people and those with abnormal kidney function.
Ghrelin is a 28 amino acid peptide hormone, which is primarily produced by the stomach, (Fig. 1).16 Ghrelin has an octanoyl group on the serine at the third position in the amino acid chain which gives the peptide hormone its biological activity.17

Figure 1: Structure of the 28-amino-acid ghrelin molecule with octanoyl modification at Ser-317
Data on ghrelin levels in women with PCOS are rather conflicting; both decreased,18-20 and elevated,21 concentrations have been reported, while others have found no significant differences between women with the syndrome and normal ovulatory women.22 Omentin-1 is a new type of Ca2+-dependent lectin with affinity for galacto furanosyl residues (the last are constituents of pathogens and dominant inmunogens).23 It is suggested, therefore, that a biological function of omentin-1 is the specific recognition of pathogens and bacterial components, an important role in the innate immune response to parasite infection.24 A study has been shown that omentin-1 is decreased in patients with PCOS.25 Glucose and insulin negatively regulate Omentin-1 levels ex-vivo and in vivo.25 Therefore, omentin-1 levels may be predictive of the metabolic consequences or co-morbidities associated with obesity.
METHODS
After overnight fasting, venous blood samples were aspirated at 08:00-10:00 am during the third and sixth days of the menstrual cycle (early follicular phase) for those of normal cycle. For patients with an ovulation or oligomenorrhea, blood samples were collected regardless of the duration of the cycle. The samples were transferred into clean plain tubes and centrifuged within 30 minutes of collection, then serum from all blood samples were separated, sugar, lipid profile levels were measured using the enzyme colorimetric methods, the rest was stored at -18 0C until the time of assay.
60 infertile Iraqi women aged 18-37 years (M±SD: 26.45±4.65) were studied. The medical history was taken, body weight and height were measured and body mass index (BMI) was calculated [mean BMI (34.01±3.54 Kg/m2)]. PCOS patients had been already diagnosed and the diagnosis had been confirmed according to the European society of human reproduction and embryology and American society for reproductive medicine criteria. PCOS is diagnosed if there are any two of the following; a) presence of polycystic ovary on ultrasound examination, b) clinical or biochemical hyperandrogenemia, and c) menstrual dysfunction with an ovulation.
The 60 PCOS women were re-evaluated after three months of treatment with 850 mg metformin twice daily. Body Mass Index (BMI) was calculated as kg/m2 and waist-to-hip ratio (WHR) was determined in all the subjects. Omentin-1, ghrelin and insulin hormones were determined by ELISA methods. Serum fasting glucose and lipid profile levels were measured by enzymatic method supplied by Giesse Diagnostic.
The homeostasis model assessment (HOMA) was used to calculate an index of insulin resistance for each patient, and beta-cell function was estimated from fasting insulin and glucose levels by the homeostasis model assessment (HOMA-ß) using fasting glucose in mmol/L and insulin in µIU/ml, the index for insulin resistance, HOMA IR, was defined as (insulin ×glucose) /22.5 and HOMA ß-cell function × 20 × insulin/(glucose-3.5).26
All statistical analysis in the study was performed using SPSS version 15.0 for Windows (Statistical Package for Social Science, Inc., Chicago, IL, USA). Descriptive analysis was used to show the mean and standard deviation of variables. The significance of differences between mean values was estimated by Student t-test. The probability p<0.05 was considered statistically significant, while p>0.05 was considered statistically insignificant. Correlation analysis was used to test the linear relationship between the parameters. ANOVA test was used to show the differences between variables of different groups.
RESULTS
In a PCOS subjects treated with metformin, the mean BMI and WHR had significantly decreased (p<0.005) and (p<0.01) respectively after 3 months as shown in Table 1. Serum levels of HDL-cholesterol were increased and the total cholesterol/HDL-cholesterol ratio was significantly decreased, (p<0.05) after three months of treatment. Serum concentrations of insulin, HOMA and HOMA ß-cell % were significantly decreased (p< 0.05), (p< 0.01) and (p<0.05) respectively after three months of treatment. In PCOS women treated with metformin for three months, serum ghrelin levels significantly increased (p<0.01) as shown in Table 1.
Table 1: The effects of metformin in PCOS patients on clinical and metabolic parameters and on concentrations of lipid profile, ghrelin and omentin1.
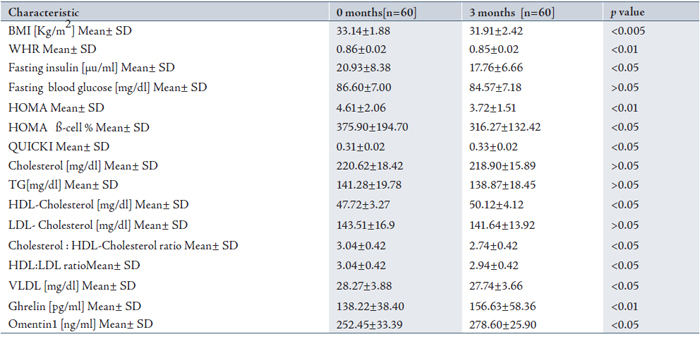
Serum levels of omentin1 were significantly increased (p<0.05) after three months of treatment, (Table 1). It is to best of the authors’ knowledge that the effects of treatment on serum omentin1 level in the present study are shown for the first time. In the present study, the effects of metformin on several parameters that depend on the omentin1 ratios were observed. (Table 2)
Table 2: The effects of metformin in PCOS patients on the omentin1 ratios
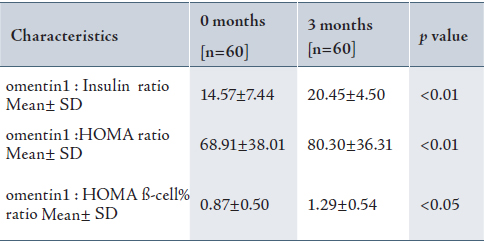
Omentin-1(ng/ml) was negatively correlated with BMI (r=-0.435, p<0.001), insulin (r=-0.424, p<0.001), HOMA (r=-0.415, p<0.001), HOMA ß-cell% (r=-0.358, p<0.05), and LDL-cholesterol (r=-0.590, p<0.05) in patients, while no significant correlation was found between omentin-1 and these factors in the controls. A significant positive correlation was found between omentin-1 (ng/ml) and QUICKI (r=0.497, p<0.001), and ghrelin (r= 0.511, p<0.001). (Table 3)
Table 3: Baseline Pearson correlations coefficients of omentin-1 levels with various metabolic and hormonal parameters in patients with PCOS.
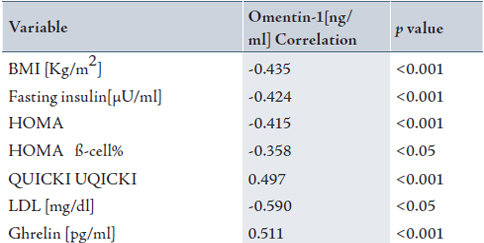
Table 4 shows a significant negative correlation between ghrelin (pg/ml) with BMI (r=-0.665, p<0.001), insulin (r=-0.897, p<0.001), HOMA (r=-0.871, p<0.001), HOMA ß-cell% (r=-0.683, p<0.001), cholesterol (r=-0.641, p<0.01), TG (r=-0.443, p<0.01), LDL-cholesterol (r=-0.656, p<0.01), LDL:HDL ratio (r=-0.643,p<0.01), and Cholesterol / HDL ratio (r=-0.638, p<0.01) in patients, while no significant correlation was found in these factors in the control group.
A significant positive correlation was found between ghrelin (pg/ml) and QUICKI (r=0.851, p<0.001). (Table 4)
Table 4: Baseline Pearson correlations coefficients of ghrelin levels with various metabolic and hormonal parameters in patients with PCOS.
DISCUSSION
In line with the study results, a dose-response effect of metformin was noticed more than 10 years ago, when it became available in the USA, and since then, the metformin dosage has been increased, with better outcomes.27
The present results are in line with those of previous studies on PCOS, where metformin has been shown to improve the lipid profile, mainly by increasing serum HDL-cholesterol concentrations.28,29 On the other hand, some other studies have shown only a negligible or no effect on lipids in women with PCOS.30,31 The mechanisms by which metformin improves the lipid profile are not clear.
Metformin has been suggested to reduce lipid uptake or synthesis in the intestine and in the hepatocytes.32 As observed in previous studies, the improvement of obesity, especially abdominal obesity, with a subsequent decreased release of free fatty acids (FFA) from adipose tissue observed during metformin therapy could also partly explain the improvement of lipid profile during metformin treatment, at least in obese women.33
Serum concentrations of insulin, HOMA and HOMA ß-cell% were decreased significantly (p<0.05), (p<0.01), (p<0.05) respectively after three months of treatment, (Table 1). The authors suggest that the reduction in hyperinsulinemia observed may be due to improvements in hepatic extraction and insulin sensitivity during therapy with metformin. They also found that metformin reduced basal levels of FFA, which as mentioned above, have a role in reducing glucose disposal in skeletal muscle, leading to impaired insulin sensitivity. Because the waist-to-hip ratio of the patients decreased after therapy, they concluded that the reduction in IR induced by metformin was secondary to its effects on adipose tissue.34 Two original papers investigating the effect of metformin on IR in women with PCOS reported negative results.35 The reasons for the partly conflicting results are not clear. A possible explanation is that only around 50% of women with PCOS respond to metformin and this could be why some studies did not report benefits from metformin therapy.
In PCOS women treated with metformin for three months, serum ghrelin levels significantly increased. Moghetti et al. in 2000, found that insulin-resistant PCOS patients treated with metformin showed improved insulin sensitivity in PCOS patients and increased serum ghrelin levels.28 This suggests a link between insulin sensitivity and ghrelin concentrations and shows that the dysregulated ghrelin system in insulin- resistant PCOS women could be normalized by metformin therapy.
Increased in omentin1 level after treatment with metformin may be due to the negative correlation between omentin 1 plasma levels with HOMA, BMI and insulin. These data suggests that some aspect of obesity negatively regulates omentin expression and release into the circulation. In the current study, treatment with metformin significantly decreased both insulin and BMI, so the increase in omentin1 level may be as a result of it. Obesity itself which may contribute in the regulation of the role of omentin in human physiology.36-38 Consequently, weight loss could be a modulator of omentin expression and function. No previous studies have showed these results. A more detailed research to study the effect of metformin on other cytokines and connection with omentin1 should be conducted.
CONCLUSION
The results from this study showed a significant increase in omentin1: insulin ratio, omentin1: HOMA ratio and omentin1; HOMA ß-cell% ratio. These results in the present study are shown for the first time to the best of our knowledge, thus suggesting that these factors may be useful in following improvements in insulin sensitivity in subjects with PCOS or obesity treated with insulin sensitizers.
However, further studies are needed to validate this measure in other populations with this treatment or with other insulin sensitizers or in patients treated with diet and exercise.
ACKNOWLEDGEMENTS
The authors reported no conflict of interest and no funding was received on this work.
|