|
Introduction
Gesstational diabetes mellitus (GDM) is defined as glucose intolerance diagnosed for the first time during pregnancy and usually disappears during the puerperium. The prevalence of GDM in some ethnic groups ranges from 1 to 14% depending on different screening methods, diagnostic criteria and the population screened.1,2 Most women who have GDM give birth to healthy neonates, especially when their blood glucose levels are well controlled with a diabetic diet, exercise and an appropriate body weight. In some cases, GDM can negatively affect the pregnancy and result in adverse perinatal outcome like macrosomia, birth trauma, shoulder dystocia and higher rates of cesarean section (CS).3,4
The management of GDM has altered markedly in recent years.3 It is based on universal screening of blood sugar and to establish a tight control of serum glucose levels round the clock in these patients through serial measurements of blood glucose by home monitoring and glycosylated hemoglobin. Adequate control of blood sugar has been associated with improved perinatal outcome. More than three-quarters of the patients with GDM respond to diet therapy alone and the remaining patients require the addition of insulin with diet.
The aim of this study was to assess the hospital incidence of GDM and maternal and fetal outcomes in pregnancies complicated by GDM compared with non-diabetic pregnancies managed at a tertiary care unit.
Methods
A retrospective study conducted on 220 patients with GDM who were diagnosed and treated at King Fahad hospital, Dammam University, Saudi Arabia, between January 2001 and December 2008. Antenatal and perinatal data obtained from the patients’ medical records and hospital database included: age, parity, BMI, gestational age at delivery, antenatal complications, mode of delivery, and birth weight of the baby, as well as maternal and neonatal morbidity and mortality. The control group consisted of 220 non-diabetic pregnant women who were not classified in the medical record coding system as having GDM, were randomly selected from the obstetric patients that matched for age, parity and BMI, who delivered in the hospital during the study period. Women who had multiple pregnancies and breech presentation in labor were excluded from the analysis. The neonatal outcomes included: birth weight at delivery; respiratory distress syndrome (RDS); hypoglycemia (<45 mg/dL); hypocalcemia (<9 mg/dL); hyperbilirubinemia (<12 mg/dL; these levels apply to term babies); and neonatal intensive care unit (NICU) admission for >24 hours. Apgar scores at 1, 5 and 10 minutes were noted from the delivery records. Neonates with jaundice (serum bilirubin ≥12 g/dL) were treated with phototherapy.
According to the departmental policy, pregnant women attending the antenatal clinics during the study period were tested for GDM by a selective screening procedure based on the following risk factors: age ≥30 years; 20% pre-pregnancy overweight; family history of diabetes mellitus; GDM in a previous pregnancy; previous unexplained stillbirth or neonatal death; previous delivery of a macrosomic baby (birth weight >4 kg); and glycosuria in ≥2 antenatal visits.
Screening for GDM was performed between 20 and 24 weeks of gestation by a 50 g - glucose challenge test given orally to the patient at any time of the day, with serum glucose measured one hour later. If the one-hour value was ≥140 mg/dL; the patient underwent a 3-hour, 100 g - oral glucose tolerance test (OGTT). GDM was diagnosed if ≥2 values met or exceeded the following cut-off point: fasting blood sugar - 100 mg/dL; 1-hr - 190 mg/dL; 2-hrs - 165 mg/ dL; and 3-hrs - 145 mg/dL; also an OGTT performed 6 weeks post delivery was within the cut-off point. Plasma glucose was obtained by venipuncture and analyzed using the glucose oxidase method.
Patients diagnosed to have GDM were put on an 1800-kcal diabetic diet for 5 days followed by a blood sugar profile (BSP) to measure the fasting blood sugar and 2-hrs postprandial-breakfast, lunch and dinner serum glucose levels. If the fasting blood sugar was ≤100 mg/dL and the postprandial blood sugar levels <125 mg/dL; the patients were managed by diet alone. Patients with higher values were treated with subcutaneous injections of regular and NPH insulin, twice daily (half an hour before breakfast and dinner). Control of blood sugar levels was monitored by bi-weekly BSP. There were 171 GDM patients treated with the diabetic diet alone, and 49 required additional insulin, besides the diet.
The patients were seen every two weeks and USG examinations were performed every 4 weeks from the time of diagnosis. Labor was induced at 40 weeks in the GDM patients controlled on diet alone without any pregnancy complication, if spontaneous onset had not occurred. Some patients required earlier induction of labor due to pre-eclamptic toxemia and poor biophysical profile. Blood sugar was measured in the newborns of diabetic mothers 30 minutes after delivery. In cases of hypoglycemia, measurements were repeated every two hours until stable values of ≥2.5 mmol were obtained. The hypoglycemic babies were treated with intravenous infusion of glucose, and breast feeding or formula was initiated as early as possible. In the control group, blood glucose was measured only when indicated by the clinical condition of the newborn.
Statistical analysis was performed by a commercial package program (SPSS 17, Chicago, Illinois). Chi-square test was performed to assess the statistical significance by the Fisher’s exact test. Odds ratio (OR) and 95% confidence intervals (CI) were calculated. All p values were two-tailed and values of ≤0.05 were considered significant. The results are given as mean ± standard deviation (SD) for normally distributed data and as frequencies (n) and percentages (%) for nominal data. To assess the independent effect of the risk factors attributing to GDM and to evaluate the independent effect of GDM on the maternal and neonatal outcomes; multivariate logistic regression was used to estimate adjusted OR and CI. The model included the significant variables found. Statistical analysis was performed using SPSS 16 (SPSS Inc, Chicago, IL). The study was approved by the hospital Health Research and Ethics review board.
Results
Among the 8,075 deliveries that occurred during the period of study, 220 (2.7%) of them were complicated with GDM. The demography and pregnancy outcome of women in the two groups are presented in Table 1. The mean gestational age at delivery was significantly different in the two groups of patients, as was history of GDM in a previous pregnancy. Statistically significant differences in pregnancy complications between the study patients and control noted were: hypertensive disorders (p<0.0001); preterm delivery (p=0.0226); induction of labor (p<0.0001); and CS rate (24.1% vs.12.3%; p<0.0019), which were also high risk variables found on multivariate logistic regression. After the adjustment for confounders, multivariate logistic analysis finally indicated that women who had a history of GDM in the previous pregnancies were at higher risk of having GDM.
The neonatal outcomes are shown in Table 2. Neonates born to women with GDM had a significantly higher mean birth weight than babies born of mothers from the control group (p<0.0001); the neonates were also large for gestational age (LGA) babies (p=0.0011) and macrosomic (birth weight ≥4000 g) compared with the neonates born to mothers from the control group. Approximately 16.4% of babies delivered by GDM mothers were admitted to the NICU for >24 hours compared to 5.5% in the control group (p=0.0003). After adjusting for potential confounding variables as listed in Table 1; infants born to mothers with GDM were at higher risk of macrosomia or being large for gestational age (Table 2). The incidence of neonatal congenital anomalies and perinatal mortality rate was similar to the controls.
There was no difference in the rates of maternal and neonatal complications (including neonatal macrosomia) in GDM mothers treated with and without insulin.
Table 1: Maternal characteristics and pregnancy outcomes in the groups.
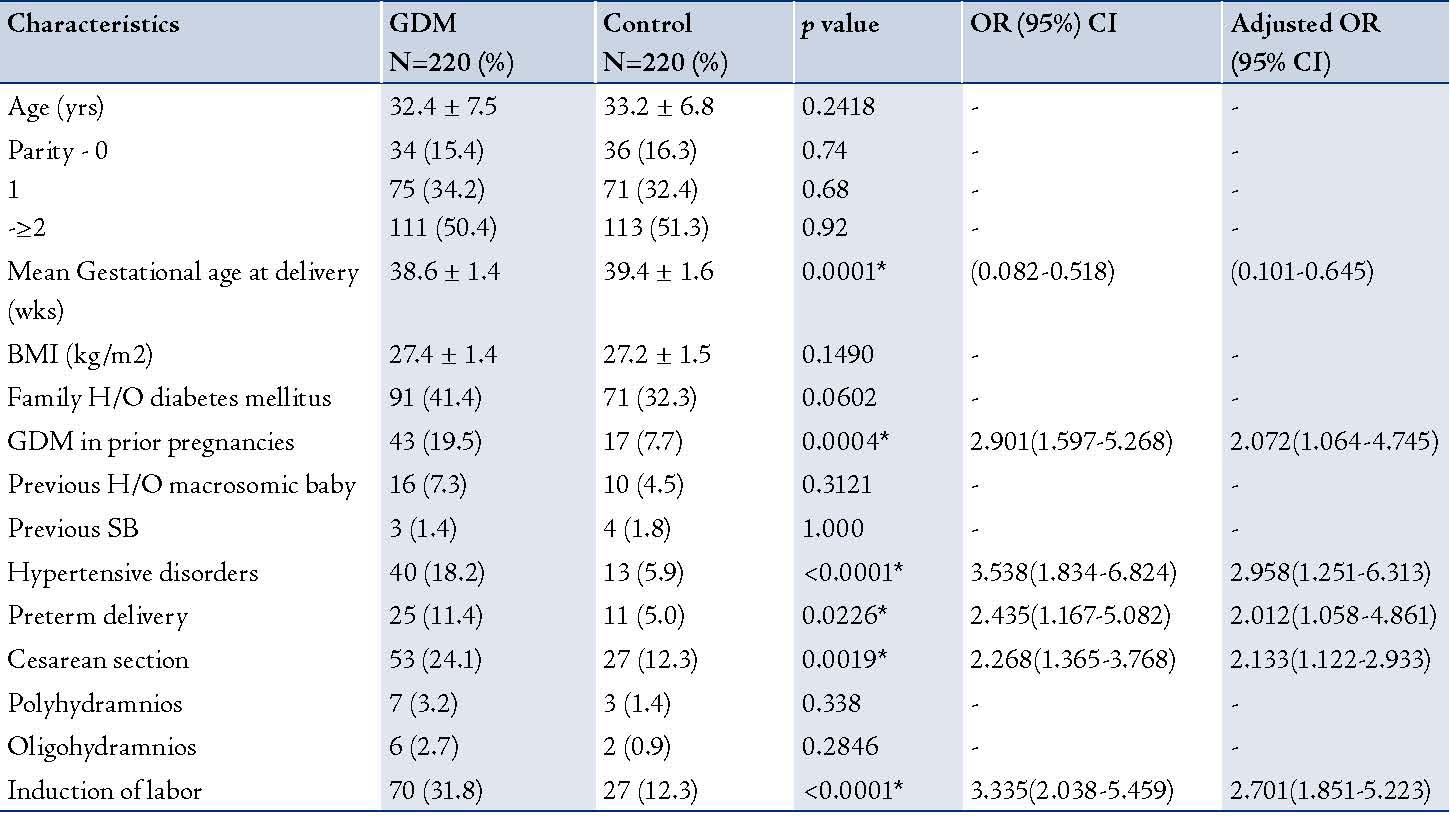
- Statistically significant, SB -Stillbirth
Table 2: Neonatal outcome between the two groups.
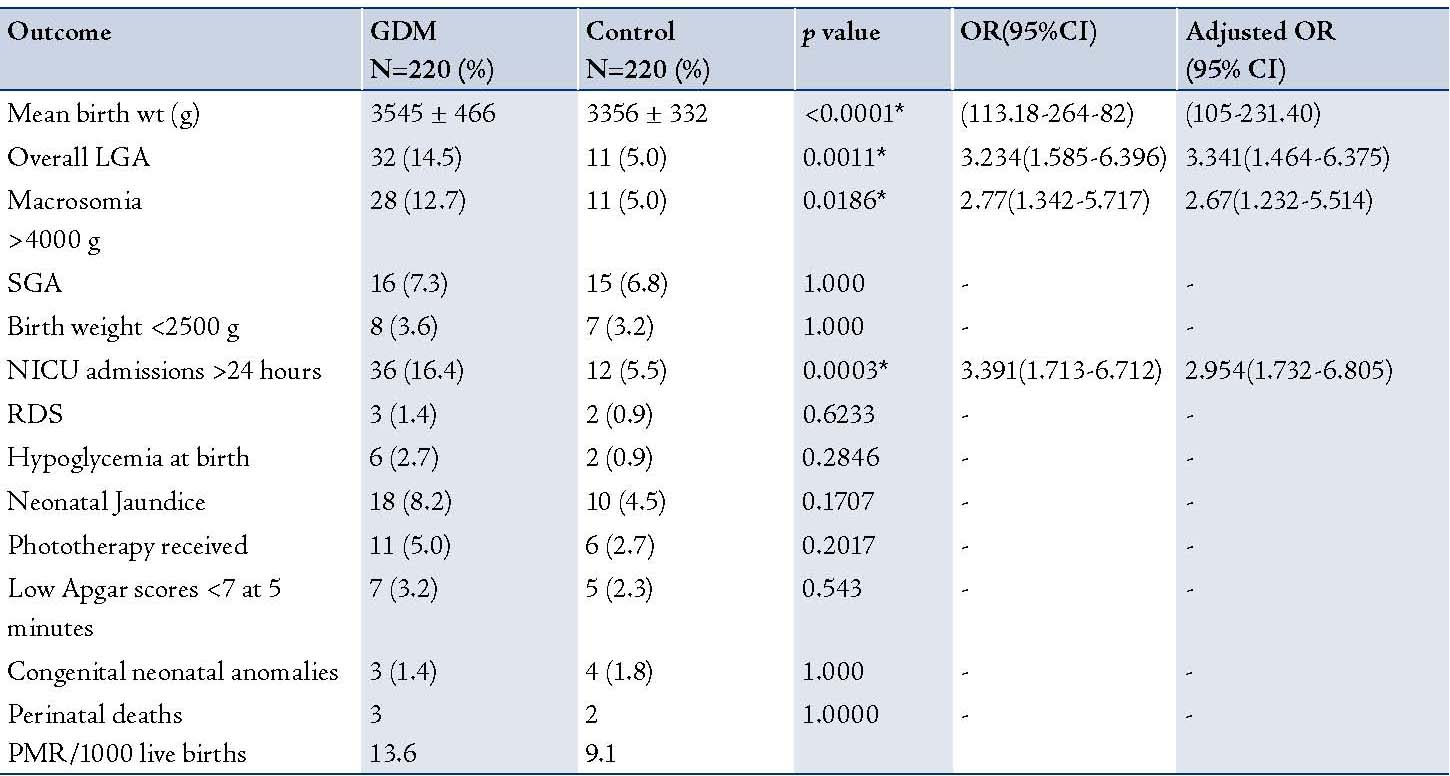
* - Statistically significant; NS - Not significan; LGA - Large for gestational age; RDS - Respiratory distress syndrome; PMR - Perinatal mortality rate.
Discussion
GDM has been recognized as a clinical entity for the past 50 years.5 It is associated with a high risk of type 2 diabetes mellitus developing in the patients later on in life, depending on the ethnicity and length of follow-up.6-9 Early studies have strongly indicated untreated carbohydrate intolerance during pregnancy to be associated with higher rates of maternal morbidity and perinatal morbidity and mortality.10-12 The purpose of screening, treatment and management of GDM is to prevent stillbirth, and decrease the incidence of LGA babies, thereby reducing maternal and perinatal morbidity and mortality. The occurrence of LGA babies is not necessarily attributable to abnormal glycemic control. Maternal age, parity, ethnicity and obesity along with fetal hyperglycemia are possible contributory risk factors for excessive fetal growth.13-16
The findings of the present study conform to those of other studies reported in the literature, that GDM patients are liable to have adverse pregnancy outcomes.3,16-18 As expected, women with GDM in the present study were found to have a higher proportion of obstetric complications including pre-eclampsia, preterm labor and CS, as well as mean birth weight, LGA and macrosomic babies than the controls.
High rates of labor induction (33-38%) among GDM patients have been reported by other authors in the past,19,20 which reflect the findings in this study (31.8%). The common indications for induction in this study were pre-eclampsia; undelivered at 40 weeks gestation controlled on diet alone, with no complication; patients who required insulin intervention; premature rupture of membranes; and maternal-related causes. Many studies have found high cesarean delivery rates in GDM patients despite good maternal blood glucose control during pregnancy.3,5,15,17,19,20 The significantly higher rate of CS in the GDM patients compared to the controls, reflect the findings of this study. The main indications for CS in this study were maternal hypertension, macrosomia, non-reassuring fetal heart tracing, failure to progress and previous history of cesarean sections. The significantly higher CS rates in the GDM patients than the controls conform to this study. The CS rate of 24.1% in this series correlates with 19-30% reported in previous studies,18,20,21 but lower than 32.9-41.4% found in some reports.3,5,15 The higher labor induction rate in the GDM patients may have had a small contribution to the increased cesarean deliveries in this series; although the cesarean section rate is not unusually high compared with other reports in the literature.
Some authors have reported that serious perinatal morbidity can be reduced with treatment of the mothers with GDM.15,16,21 Published, randomized clinical trials confirm that treating pregnant patients with even the mildest form of GDM can reduce the risk of common birth complications among the infants and blood pressure disorders in the mothers.22,23
The rate of pregnancy complications in the study was similar among the GDM patients treated with diet alone and those who received additional insulin alongside the diet, which correlated with the findings of some reports.15,21 Significantly higher rates of preterm delivery and admission of babies to the NICU have been reported in the GDM patients treated with insulin and diet compared with those on diet alone,16,24 which were contrary to the findings in this and other series.3,5
Many complications of pregnancy that are commonly associated with GDM such as polyhydramnios, oligohydramnios, SGA neonates, neonatal hypoglycemia and those requiring phototherapy were not significantly increased in the patient group of this study compared with the control.
The incidence of 16.4% of neonates of GDM mothers admitted to the NICU in this study was significantly higher than the control (p<0.0003). Although, the Apgar scores were not strikingly different between the two groups studied; babies born to GDM mothers spent significantly more time in the NICU than babies born to mothers from the control group. This may reflect the routine policy of observation of these infants at the hospital where this study was based and not necessarily associated with any medical problems. In GDM, increased numbers of pregnancy risk factors and fetal complications appear to cause significant numbers of NICU admissions >24 hours. The rate of NICU admission (16.4%) in the study for GDM neonates was lower than 28.7% reported in one study.18
Some studies concluded that even very mild alterations in glucose tolerance can result in abnormal fetal growth which can be prevented by simple but aggressive control of blood sugars in order to ameliorate many of the complications for the mother and the baby.22,23 Dietary intervention and insulin therapy, with their safety profile, have been considered the gold standard of pharmacotherapy for GDM. On the other hand, a number of trials, including prospective randomized trials, have demonstrated the efficacy of oral hypoglycemic agents, particularly glyburide and metformin, used in managing pregnant diabetics.24 Furthermore, a short-term study has not shown any adverse effect of these oral medications on the fetus, which are increasingly being used in pregnancy.25
Conclusion
Outcomes of pregnancy in women with GDM in this study showed significantly raised incidences of hypertensive disorders, CS, LGA neonates, macrosomia and NICU admissions for >24 hours compared with the non-diabetic mothers who delivered at the hospital. These findings support the paradigm of increased rates of some maternal and neonatal complications in pregnant women with GDM. There is strong evidence which suggests that the reduction of complications can be significantly achieved by aggressive treatment of GDM.12
It is likely that GDM will continue to pose a problem for pregnant women, due to the rising incidence of obesity worldwide and a predisposition to the development of Type 2 diabetes mellitus later in life.4 The fact that even mild GDM seems to have significant consequences for women and their babies; it has been recommended to screen for GDM. However, the major questions remaining include: (a) what is the best screening method, (b) when to begin the screening in pregnancy, and (c) at what level of hyperglycemia should aggressive intervention be initiated. Currently, various screening and diagnostic tests are used to diagnose GDM but none of them off offer the combination of qualities to be expected from a test: simplicity of use, reproducibility, specificity and sensitivity.12
A multicenter, randomized controlled trial (RTC), with a universal GDM screening by a glucose challenge test and a standardized diagnostic OGTT with clearly defined and universally accepted cut-off values for the screening as the gold standard test is needed. All patients with GDM, defined by universally accepted criteria, should be randomized to a diabetic diet and intervention with insulin or metformin, followed by self monitoring of glucose at standardized times, with standardized cut-off glucose levels for intervention. This RCT would not only be extremely difficult to design and conduct to precision, but also to obtain appropriate financial support for the project.
Acknowledgements
The author reported no conflict of interest and no financial support was granted for this work.
References
1. Position Statement AD; American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2004 Jan;27(Suppl 1):S88-S90.
2. Karcaaltincaba D, Kandemir O, Yalvac S, Güvendag-Guven S, Haberal A. Prevalence of gestational diabetes mellitus and gestational impaired glucose tolerance in pregnant women evaluated by National Diabetes Data Group and Carpenter and Coustan criteria. Int J Gynaecol Obstet 2009 Sep;106(3):246-249.
3. Sendag F, Terek MC, Itil IM, Oztekin K, Bilgin O. Maternal and perinatal outcomes in women with gestational diabetes mellitus as compared to nondiabetic controls. J Reprod Med 2001 Dec;46(12):1057-1062.
4. Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med 2010 Mar;23(3):199-203.
5. Jensen DM, Sørensen B, Feilberg-Jørgensen N, Westergaard JG, Beck-Nielsen H. Maternal and perinatal outcomes in 143 Danish women with gestational diabetes mellitus and 143 controls with a similar risk profile. Diabet Med 2000 Apr;17(4):281-286.
6. O’Sullivan JB. Diabetes mellitus after GDM. Diabetes 1991 Dec;40(Suppl 2):131-135.
7. Damm P, Kühl C, Bertelsen A, Mølsted-Pedersen L. Predictive factors for the development of diabetes in women with previous gestational diabetes mellitus. Am J Obstet Gynecol 1992 Sep;167(3):607-616.
8. Kjos SL, Peters RK, Xiang A, Henry OA, Montoro M, Buchanan TA. Predicting future diabetes in Latino women with gestational diabetes. Utility of early postpartum glucose tolerance testing. Diabetes 1995 May;44(5):586-591.
9. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002 Oct;25(10):1862-1868.
10. Turok DK, Ratcliffe SD, Baxley EG. Management of gestational diabetes mellitus. Am Fam Physician 2003 Nov;68(9):1767-1772.
11. Reece EA, Homko C, Miodovnik M, Langer O. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Arkansas, May 2002. J Matern Fetal Neonatal Med 2002 Dec;12(6):362-364.
12. Langer O, Miodovnik M, Reece EA, Rosenn BM. The proceedings of the diabetes in pregnancy study group of North America 2009 conference. The J Matern-Fetal Neonat Med 2010; 23(3):196-198
13. Casey BM, Lucas MJ, Mcintire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynecol 1997 Dec;90(6):869-873.
14. Seghieri G, Anichini R, De Bellis A, Alviggi L, Franconi F, Breschi MC. Relationship between gestational diabetes mellitus and low maternal birth weight. Diabetes Care 2002 Oct;25(10):1761-1765.
15. Johns K, Olynik C, Mase R, Kreisman S, Tildesley H. Gestational diabetes mellitus outcome in 394 patients. J Obstet Gynaecol Can 2006 Feb;28(2):122-127.
16. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS; Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005 Jun;352(24):2477-2486.
17. Jimenez-Moleon JJ, Bueno-Cavanillas A, Luna-del-Castillo J, Gracia-Martin M, Lardelli-Claret P, Galves-Vargas R. Impact of different levels of carbohydrate intolerance on neonatal outcomes classically associated with gestaional diabetes mellitus. Eur J Obstet Gynecol Reprod Biol 2001;102:36-41 .
18. Ostlund I, Hanson U, Björklund A, Hjertberg R, Eva N, Nordlander E, et al. Maternal and fetal outcomes if gestational impaired glucose tolerance is not treated. Diabetes Care 2003 Jul;26(7):2107-2111.
19. Peticca P, Keely EJ, Walker MC, Yang Q, Bottomley J. Pregnancy outcomes in diabetes subtypes: how do they compare? A province-based study of Ontario, 2005-2006. J Obstet Gynaecol Can 2009 Jun;31(6):487-496.
20. Tan PC, Ling LP, Omar SZ. The 50-g glucose challenge test and pregnancy outcome in a multiethnic Asian population at high risk for gestational diabetes. Int J Gynaecol Obstet 2009 Apr;105(1):50-55.
21. Xiong X, Saunders LD, Wang FL, Demianczuk NN. Gestational diabetes mellitus: prevalence, risk factors, maternal and infant outcomes. Int J Gynaecol Obstet 2001 Dec;75(3):221-228.
22. Bonomo M, Corica D, Mion E, Gonçalves D, Motta G, Merati R, et al. Evaluating the therapeutic approach in pregnancies complicated by borderline glucose intolerance: a randomized clinical trial. Diabet Med 2005 Nov;22(11):1536-1541.
23. Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009 Oct;361(14):1339-1348.
24. Paglia MJ, Coustan DR. The use of oral antidiabetic medications in gestational diabetes mellitus. Curr Diab Rep 2009 Aug;9(4):287-290.
25. Serlin DC, Lash RW. Diagnosis and management of gestational diabetes mellitus. Am Fam Physician 2009 Jul;80(1):57-62.
|